| Pages:
1
2 |
thereelstory
Harmless

Posts: 41
Registered: 18-1-2010
Member Is Offline
Mood: No Mood
|
|
synthesis of formaldehyde?
could someone please give me some insight on how to prepare this?
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Heat some pure copper wire til red heat, quench into a beaker of methanol. With each quenching some formaldehyde is produced, keep repeating until it
is at desired concentration, may require cooling. Otherwise heat a section of copper tube til red, run methanol vapour through, significant fire and
poisoning risk.
I may know your brother, the unrealstory. Funny joke, Ha!
[Edited on 20-1-2010 by Panache]
|
|
|
medchem
Harmless

Posts: 41
Registered: 12-12-2009
Member Is Offline
Mood: No Mood
|
|
you can try catalytic (silver) oxidation of methanol
following paper has included other methods too, plz refer to it.....
Applied Catalysis A: General
Volume 238, Issue 2, 20 January 2003, Pages 211-222
good luck
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Pyrolysis of formate salts (for example calcium or zinc) will give some formaldehyde, but I believe yields are poor.
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
You can decompose trioxane fuel tablets with sulfuric acid as a catalyst; trioxane is a trimer of formaldehyde and is broken down under strongly
acidic conditions. The fuel tablets can be gotten for very cheap as military surplus, however they have a waxy binder which inhibits the reaction
making it a somewhat slow process. It would be ideal if this could be separated but I have not had much success doing that.
The mind cannot decide the truth; it can only find the truth.
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by S.C. Wack  | PREPARATION OF FORMALDEHYDE
In the usual way.
See original post for attachment here
Saw the name H. Adkins in Blazter's document and figured that this is a non-bullshit patent.
US19330613
[Edited on 2-8-2009 by S.C. Wack] |
|
|
|
bbartlog
International Hazard
    
Posts: 1139
Registered: 27-8-2009
Location: Unmoored in time
Member Is Offline
Mood: No Mood
|
|
Now, *that* is a patent. If all patents contained that level of detail and restricted themselves to such a narrow range of claims, I'd be a lot more
enthusiastic about our IP system. Instead most patents I see now (specifically relating to catalysts) try to define some huge space of compounds
(XaYbZc where X is a group blahblahblah metal and Z is one of O, S, or SO4 and a b c are in the range of 0.1 to 2.7 and etc...), such that it's clear
that the applicant can't possibly have thoroughly explored the space and is just trying to stake a claim.
[Edited on 20-1-2010 by bbartlog]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
There has been continued interest here in production of Formaldehyde
http://www.sciencemadness.org/talk/viewthread.php?tid=132
http://www.sciencemadness.org/talk/viewthread.php?tid=525
http://www.sciencemadness.org/talk/viewthread.php?tid=5993
The established method of production is by partial oxidation of Methanol vapor
in air against a silver catalyst at 450 - 650 ºC , yielding formalin
2 CH3OH + O2 => 2 CH2O + 2 H2O , containing unreacted methanol.
The following known pathways suggest another method of preparative value.
An electric arc passed between carbon electrodes under water consumes
the electrodes C + H2O => CO + H2 forming water gas.
A mixture of CO + H2 under the action of dark (silent) electric discharge
yields formaldehyde => CH2O
The first process has been known a long time , it's mentioned on page 485
as a Preparation of Carbon Monoxide in
" A Treatise on General and Industrial Inorganic Chemistry " 1920
http://books.google.com/books/download/Treatise_on_general_a...
More recently rediscovered as a means of coal gasification , the important thing
is not the claims made by the promoter , but that the production of CO + H2 is
plentiful and abundant depending only on the amount of electric power consumed.
http://www.blazelabs.com/n-aquagen.asp
http://www.blazelabs.com/n-newfuel.asp
The next process combining CO + H2 into Formaldehyde is somewhat obscure.
It is simply an ozonizer aparatus which is instead infused with CO and H2.
Seemingly , no one realized the water gas process complements co-generation.
This is difficult to make right , results are mixed , only some have seen success.
Interaction of Carbon Monoxide and Hydrogen Under Silent Discharge:
Production of Formaldehyde - http://www.springerlink.com/content/q2k5754wk726t676
http://resources.metapress.com/pdf-preview.axd?code=q2k5754w...
http://www.fischer-tropsch.org/Bureau_of_Mines/abs_of_lit/li...
http://www.fischer-tropsch.org/Bureau_of_Mines/abs_of_lit/li...
http://www.fischer-tropsch.org/Bureau_of_Mines/abs_of_lit/li...
Losanitsch and Jovitschitsch , Ber., (1897) 30, pg 135
Löb , Z. Elektrochem., (1906) 12, pg 282
Koenig and Weinig _ http://www.fischer-tropsch.org/Bureau_of_Mines/abs_of_lit/li...
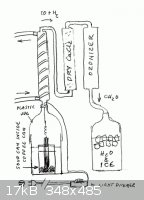
A basic setup would be a carbon rod greased with vaseline to make it electrically
insulated , standing in the open end of a metal can in proximity to perhaps 40 cm
of granular metalurgical coke in the bottom , submerged in a half liter of water. This
can stands inside another canister filled with water running into it to provide cooling.
The can is grounded corresponding to the electric socket ground and the carbon rod
is the hot wire. Electric power is regulated by a small choke in series with the circuit.
A cutoff plastic soda bottle top fits over the inside can so that it is air tight. The
opening runs the gas produced into a chilled reflux column intended to stop water
vapor. The gas is further dried by passing through a container of Calcium Chloride
" ice melt salt ".
The dry gas now enters the " ozonizer " to be converted into Formaldehyde , from
there directly absorbed into a receiver holding perhaps a liter of chilled water. The
entire device will have been initially purged of all air containing only water gas. As
the Formaldehyde formed is continuously dissolved providing a partial vacuum , the
positive pressure of the water gas formed drives the flow toward the reciever. The
beauty of this is that no metering of the CO , H2 gas is required since it is produced
quantitatively in the exact proportion necessary at the source. The gas flow must
be turbulent to prevent separation , as Hydrogen is less dense than CO.
If one wants to produce hexamine directly , the receiving liquor can contain a
solution of Ammonium Sulfate , which should precipitate Hexamine di-sulfate.
Important - Carbon monoxide being an odorless poison gas requires a detector
of it's presence be close to the setup when it's run to warn of any leak. These
are available in hardware stores for around 50 - 60 dollars. Failure to take this
prcaution means you may never wake up.
.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
I've had great success in separating pure trioxane from fuel tablets. Fill bottom 1/4 of 5 gallon plastic bucket with them, snap on lid, and leave
them on a small low power heating pad, such as is used for seed germination or terrarium heating. Pure Trioxane will sublimate and crystallize on
the upper sides and top of the bucket over about a 2 week period. 
You can also melt them and strain out the wax for initial purification, but it's messy and odorous.
[Edited on 3-7-2010 by Eclectic]
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
Just theoretically: Since "al-dehyd" means "alcoholus dehydratus", i.e. dehydrated alcohol:
==> Can't just the Alcohol (Methanol) be dehydrated ?
Maybe it can't for some reason, just sharing ...
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
CH3OH - H2O --> CH2 (carbene)
CH3OH - H2 --> HCHO (formaldehyde)
ITYM dehydrogenation and not dehydration.
In any case, paraformaldehyde is trivially depolymerized to formaldehyde and the former should be available practically everywhere.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
I was asked to sketch the apparatus I proposed above
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
An electrolytic process to make formaldehyde is disclosed in this
abstract , the product is essentially commercial strength formalin.
Translated below, restating the preceding brief summary is the
excerpt from the cited German Patent Attachment: DE138442.pdf (251kB)
This file has been downloaded 930 times

Beispiel V.
Für die Bildung von Aldehyden mag nachstehendes Beispiel zur Erläuterung dienen:
Als Elektrolyt dient eine Lösung, die in 500 ccm Flüssigkeit ca. 120 g Natriumacetat
und 160 g Natriumchlorat enthält. Die Elektroden bestehen aus Platinblech, die
Stromdichte ist ca. 20 bis 30 Amp. pro Quadratdecimeter, die Temperatur ca.
20 bis 30° C. Die Isolierung geschieht wie bereits beschrieben.
Man erhält bei diesem Versuch wesentlich Formaldehyd.
Die Ausbeute an Formaldehyd und Methylalkohol beträgt etwa 60 pCt. der Theorie,
wobei beide Körper etwa im Verhältnis 2 : 1 sich gebildet hatten.
Example V.
For the formation of aldehydes the following example may serve for explanation:
A solution of 500 cubic centimetres serves as an electrolyte, containing approximately
120 gms of sodium acetate and 160 gms sodium chlorate. The electrodes are of platinum metal,
Current density is approximately 20 to 30 amperes per square decimeter, the temperature
approximately 20 to 30 °C. Isolation is as already described.
One receives with this attempt substantial formaldehyde.
The yield in formaldehyde and methyl alcohol amounts to about 60 percent of the theory,
both form possibly in the proportion 2 : 1
.
|
|
|
questions
Hazard to Others
  
Posts: 103
Registered: 12-2-2011
Location: Australia
Member Is Offline
Mood: curious
|
|
I just thought of an idea, please if anyone has any thoughts, please tell me what you think.
I add the right amount of Hcl to hexamine in a thick HDPE container so it will break the hexamine down to a solution of water, formaldehyde and
ammonium chloride. I then put in lead electrodes and begin passing electricity through the solution. I then put an air tight lid over the top of the
solution and use a vacuum pump to suck all the air out of the container. This will cause the formaldehyde to boil out and into a receiver container
with ice and chilled water to collect it.
I'm guessing that the ammonium and chlorine ions wont disstill over with the formaldehyde as they would still be bound to the electrodes as
electricity would still be passing through it.
Do you think this would work in my attempt to separate the ammonium chloride from the formaldehyde solution ?
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by questions  | I just thought of an idea, please if anyone has any thoughts, please tell me what you think.
I add the right amount of Hcl to hexamine in a thick HDPE container so it will break the hexamine down to a solution of water, formaldehyde and
ammonium chloride. I then put in lead electrodes and begin passing electricity through the solution. I then put an air tight lid over the top of the
solution and use a vacuum pump to suck all the air out of the container. This will cause the formaldehyde to boil out and into a receiver container
with ice and chilled water to collect it.
I'm guessing that the ammonium and chlorine ions wont disstill over with the formaldehyde as they would still be bound to the electrodes as
electricity would still be passing through it.
Do you think this would work in my attempt to separate the ammonium chloride from the formaldehyde solution ? |
Formaldehyde is quite a poison and i think given your question, which although imaginative shows significant inexperience and less knowledge, that you
best develop your skills with something more benign, begin perhaps with distilling water and ammonium chloride to see some of the properties of the
chemicals you want to play with.
In short, and i intend no patronization, what you propose is quite dangerous and will not work as you describe.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
I seem to remember that electrolysing a solution of ammonium chloride is one of the ways that you can make nitrogen trichloride.
Fascinating stuff, it's most notable property is that it explodes at the slightest provocation.
|
|
|
questions
Hazard to Others
  
Posts: 103
Registered: 12-2-2011
Location: Australia
Member Is Offline
Mood: curious
|
|
I am trying to make formaldehyde. When I add Hcl to hexamine, it turns into a solution of formaldehyde, water and ammonium chloride. Adding Hcl to
hexamine is a great way to get formaldehyde but I want to get rid of that that ammonium chloride that also forms, does anyone have any ideas as to how
I can somehow remove the ammonium chloride out of the solution?
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Why not buy some formadehyde?
It is readily available for treating diseases in Koi carp where I live. The local aquarium shop will sell you a litre without batting an eyelid 
|
|
|
questions
Hazard to Others
  
Posts: 103
Registered: 12-2-2011
Location: Australia
Member Is Offline
Mood: curious
|
|
formaldehyde
Here in australia the most we can buy from an aquarium is a 3.7% solution. It is just too dilute 
|
|
|
azo
Hazard to Others
  
Posts: 163
Registered: 12-2-2008
Member Is Offline
Mood: No Mood
|
|
just go to a farming warehouse and buy sheep dip that is 37% formaldehyde nothing else.
|
|
|
questions
Hazard to Others
  
Posts: 103
Registered: 12-2-2011
Location: Australia
Member Is Offline
Mood: curious
|
|
formaldehyde
I have found that alot of farming supply companies don't sell it anymore as they are starting to say that there as other substituteswhich are as good
and less dangerous to humans.
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
id be looking into the neff reaction with nitromethane.
I belive it would make for some pritty pure formaldehyde 
might be a bit pricey but thats never stopped me.
the problem you will get with using hexamine is the formation of methylamine from
imine formation between formaldehyde and ammonium chloride.
with the neff this will not happen.
still I would be using dilute sulfuric acid to work with the nitro salt.
this way when you distill you dont get HCl comming over as well.
make sure you dont use too much acid or you will polymerise the aldehyde.
[Edited on 12-4-2011 by Ephoton]
e3500 console login: root
bash-2.05#
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
camping stores in australia sell a 22% solution in one and two litre bottles as 'porta loo treatment'. Problem is when you smell it you just want to
go to a festival.
|
|
|
azo
Hazard to Others
  
Posts: 163
Registered: 12-2-2008
Member Is Offline
Mood: No Mood
|
|
Be carefull thinking that portable toilet fluid has formaldehyde in it, this is not the prefured compound these days because of the toxic properties
of formaldehyde.
glutaraldehyde is more used because it controls odours better than quaternary ammonium compounds.
regards azo
[Edited on 13-4-2011 by azo]
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by azo  | Be carefull thinking that portable toilet fluid has formaldehyde in it, this is not the prefured compound these days because of the toxic properties
of formaldehyde. is more used because it controls odours better than quaternary ammonium compounds.
regards azo
[Edited on 13-4-2011 by azo] |
Really, thats interesting, i might get some now and see, its (formaldehyde) is a schedule 7 poison since 2003 in australia and you're right using it
for anything i a regulatory headache. i know within the food industry its simply impossible and not worth the trouble, even for plant washdown during
shutdown.
|
|
|
| Pages:
1
2 |