Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Polyaniline
I have recently been doing some study on polyaniline, a promising conductive polymer. I synthesis this by adding a 25% excess of sodium persulfate
solution to a solution of aniline hydrochloride with excess HCl (about 0,5mL conc. HCl excess, 1mL of aniline was used). After stirring at about 10C
for 6 hours, I filtered of the very dark green precitipate, and washed this 2 times with 0,2M HCl and finally acetone, followed by drying at about
50-60C).
After drying I obtained some brittle pieces of polymer (very easily to crush into a powder). Because I don't have a Volt-meter at hand yet, I am
thinking of converting it in a more usable form, such as a film on an object, or a single piece of the polymer. It seems I will have to dissolve the
polymer.
This is the problem. Except for a few speciality solvents (especially Methylpyrrolidone) , it is insoluble in everthing. I have tried DCM and
chloroform without succes. I can buy methylpyrrolidone for 25 EUR 1 liter or 10 EUR 100mL, but I rather don't).
According to this patent it can be made soluble.
http://www.freepatentsonline.com/EP0455222.html
i have the hydrazine. But I don't have the alkyl halides such as bromodecane, or other relatively high alkyl halides, and I don't know where I can get
these alcohols in good purity. And it uses the solvent Methylpyrrolidone again.
Can the reaction also proceed by suspending the polyaniline in another solvents, avoiding Methylpyrrolidone?
Or is there another way to effectively make a homogenous unit of the polymer, without using these speciality solvents?
I was thinking of melting it, but I have not yet had the time of trying it (not at home now) but it will certainly decompose before melting.

[Edited on 2-1-2010 by Jor]
[Edited on 2-1-2010 by Jor]
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
Hello Jor,
As I've been doing some research on conducting polymers myself, I can give some advice. Films of conducting polyaniline (poly(emeraldine
hydrochloride)) can be obtained very easy by electrochemical polymerization onto metal electrodes (stainless steel, gold, platinum). All you need is a
dc source, a variable resistor, an ammeter and two electrodes for a simple electrochemical setup suitable for electropolymerization under
galvanoststic (i.e. controlled current) conditions. The electrolyte is a solution of aniline in diluted hydrochloric acid. Free-standing films of
polyaniline can be stripped off the electrode.
Conducting polyaniline can also be obtained as a dispersion in water by emulsion chemical polymerization in the presence of surfactants such as
dodecylbenzenesulfonic acid sodium salt (DBSA) - see for example Polymer, 1994, 35, 3902. Films of polyaniline can be obtained from the dispersion by
evaporating the solvent or spin-coating, dip-coating, etc.
Yet another option is the polymerization of other derivatives of aniline, such as N-substituted alkylanilines (N-ethylaniline, N-butylaniline), which
render polymers soluble in common solvents such as acetonitrile, dimethylformamide (Macromolecules, 1992, 25, 3325; Materials Letters, 2004, 58,
1934).
A water-soluble derivative of polyaniline - sulfonated polyaniline (SPANI) - can be obtained from polyaniline by sulfonation with oleum or
chlorosulfonic acid and then hydrolysis (US 6326441; Polymer, 1992, 33, 4410; Synthetic Metals, 1997, 85, 1337; Macromolecules, 1996, 29, 3950;
Synthetic Metals, 1998, 96, 161).
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
Here are some references:
Attachment: jor1.zip (1.2MB)
This file has been downloaded 715 times
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
Attachment: jor2.zip (1.8MB)
This file has been downloaded 703 times
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Thank you very much matei! This is very interesting chemistry.
The method using electrochemical oxidation is not very convenient for me, as i do not own all required equipment.
The second method ypou describe using the surfactant is interesting, but I do not have DBSA. Now, sodium laureth sulfate is somewhat easier to obtain.
I guess this will also work.
The polymerisation of N-alkylamines is a very interesting route. Only concern is that I will have to bring in methylating agents. But with care this
should be no problem. I can easily prepare EtBr from H2SO4/KBr/EtOH, dissolve this in some solvent and stir in the aniline. This way, I think the
hydrobromide-salt will precitipate. However, i will have to use an excess of aniline to prevent dialkylation.
I find the sulfonating of the polymer the most interesting reaction. I can buy chlorosulfonic acid. Probably i will buy 100mL of it when I put my next
order. A great thing is that the polymer would be water-soluble, so I won't need organic (toxic) solvents.
In one of your articles I read that you can acetylate the polymer in pure Ac2O at 80C to form poly(N-acetylbenzamide), wich is soluble in 'common
solvents'. Now I hope this includes either DCM, chloroform, ether, ethanol, methanol, isopropyl alcohol, as these are the only solvents I have at hand
(also CCl4 and benzene, but I rather avoid these). I guess I will need to buy some DMF sometime (very cheap). I will do some experimenting on this
N-acetylated version. maybe I'll try with trifluoroacetic anhydride as well, as the article mentions it. I have 10mL of this 
I think I will first have to make the fully reduced version of the polymer before acetylating, but I;m not sure. I read that this can be done, by
first treating the polymer with ammonia (as the polymer initially prepared contains HCl (protonated N-atoms), then reduce with hydrazine.
I can imagine this is a very exciting area to do your research!
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Jor, NMP is a very useful solvent especially if you are interested in inorganic and organometallic chemistry, and if you can get it that cheap I
recommend you to buy it as you will need it for other things as well. In organic chemistry you can often do without it and use DMF instead, but the
opposite is also commonly true and NMP can substitute DMF in most cases. So if you think you will ever need DMF you might consider to buy NMP instead.
Things that need NMP to dissolve usually dissolve in DMSO as well, so if you have some, you can try that. Though I'm quite sure the solubility of
polyaniline in DMSO is surely reported in the literature.
I'm looking forward to hear of your experiments in the synthesis of N-ethylaniline. This will however not be very easy with ethyl bromide which would
give a mixture even though it is true that fractionation would separate it easily, I think. Maybe a better alternative would be to make
N-isopropylaniline by reductive alkylation with acetone. This way you would get much less (if any) N,N-dialkylated product and a polymer more soluble
in less polar solvents. I can do a literature search if you are interested, though I think N-acetylating the polymer is a more realistic option to
start with.
PS: Simple electronic multimeters cost just a few euros. You do not need a 150EUR digital one for such a simple experiment. Those <10EUR worth
analog ones will do just fine.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Can you tell me for what kind of experiments/synthesis NMP can be used in inorganic chemistry? If it is indeed such a useful solvent, I may buy some,
together with some chlorosulfonic acid (wich i always wanted anyways). Only thing that keeps me from having the chlorosulfonic acid is the fact that i
heard it is EXTREMELY corrosive, much like oleum and such, instantly burning on contact. Is this true?
I don't have DMSO, so I can't try that.
I think I will try the N-acetylating of the polymer first, having plenty of acetic anhydride and being an easy experiment. I am also interested in
sulfonating the polymer, when i get the ClSO3H. I am not sure if I want to go the N-ethyl (or isopropyl) route, at least not soon. When I look up
(google) the first reductive alkylations of aniline with acetone, they require high pressures of hydrogen or precious metal catalysts. I don't have
these at hand. Do you know of a simpler procedure?
Only problem is that I cannot do experiments soon. It is very cold in the garage now. Water is frozen, so i have no running water, wich makes working
very impractical. And when I do experiments and have the hood running it gets even colder in the garage, giving the rabbit (wich is currently in there
for the winter) an even harder life 
Due to the cold I hardly experiment, so I have many projects on standby, like this one, but also the synthesis of Mn doped ZnS (phosphorescent) and
extraction of Mn2O7 from H2SO4 with CCl4 (this failed previous times, but it is supposed to work). I think CCl4 is actually the only solvent wich can
be inert to Mn2O7, everything else just gets oxidised.
[Edited on 4-1-2010 by Jor]
[Edited on 4-1-2010 by Jor]
|
|
|
sonogashira
National Hazard
   
Posts: 555
Registered: 10-9-2006
Member Is Offline
Mood: No Mood
|
|
Dimethyl sulfone (DMSO2), sold in health stores, is a very good polar aprotic solvent. Unfortunately it has high melting point, but it can dissolve in
many other solvent and will certainly increase the polarity (like DMSO/DMF), if that is what you desire (haven't had time to read properly - but I
shall later as this type of chemistry interests me too.)
There is also a review by Gribble I think who wrote of the high yield of aniline alkylation with carboxylic acid and NaBH4 (so acetic acid and NaBH4
for ethylation) which may be of interest too. I will find later if you can not, but it is on this website I am sure - and certainly on the internet
for free. 
[Edited on 4-1-2010 by sonogashira]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Jor  | | Can you tell me for what kind of experiments/synthesis NMP can be used in inorganic chemistry? |
I'm not an inorganic chemist. 
I have been told by an "inorganic friend" that NMP is the alternative aprotic solvent of choice used to dissolve transition metal complexes (anything
from pi, sigma to phosphine complexes of Pd, Ni, Rh, Ir...) where THF, acetonitrile and others fail. It is only used when necessary because of its
boiling point making it less easy to remove. But then again I was only asking him for this specific use so maybe I generalized too much in my claim
that it is one of the most common solvents. In organic chemistry the solubility of these noble metal complexes is just one of the many reasons to use
these types of solvents (NMP, DMF, DMSO, etc.) in organic C-C coupling reactions employing complexes of Pd, Ni, Ru, Rh and other transition metals as
catalysts (another related reason is in that these polar solvents stabilize the zerovalent metal complexes by inhibiting their decomposition to the
metal).
In short, NMP, DMF and DMSO dissolve a lot of inorganic salts that are otherwise only soluble in protic solvents, and additionally also many complexes
bearing hydrophobic ligands that are insoluble both in water and many nonpolar solvents.
| Quote: | | When I look up (google) the first reductive alkylations of aniline with acetone, they require high pressures of hydrogen or precious metal catalysts.
I don't have these at hand. Do you know of a simpler procedure? |
I think the simplest and amateur friendly synthesis of N-isopropylaniline from aniline and acetone would be the one described in Synthesis (1991) 1043-1045. Othewise, alkylation of aniline with isopropyl bromide also gives N-isopropylaniline as product in good
yields. But it would be first necessary to check if poly(N-isopropyl)aniline can be made at all. Maybe matei will be kind enough to check the
literature.
| Quote: | Only problem is that I cannot do experiments soon. It is very cold in the garage now. Water is frozen, so i have no running water, wich makes working
very impractical. And when I do experiments and have the hood running it gets even colder in the garage, giving the rabbit (wich is currently in there
for the winter) an even harder life  |
Then give experiments a rest and do get some life till spring. 
EDIT: In Materials Letters, 58 (2004) 1934–1937 cited by matei in a post above it says that polyaniline is soluble in DMSO
to an extent of 5.5 g/L (while in NMP it is 9.9 g/L). Poly(N-methylaniline) is soluble to 16.7 g/L in DMSO, 35.7 g/L in NMP, 2.1 g/L in methanol and
1.2 g/L in acetone or ethanol.
I could find no references mentioning poly(N-isopropylaniline) or any other poly(N-isoalkylaniline) whatsoever, so either I did not find it, it can
not be made or just nobody ever made it. I wander if it would be conductive at all since such a bulky group would make it near to impossible for a
planar alignment of the aryl-N-aryl structures.
Just as a curiosity, according to Synthetic Metals, 92 (1998) 39-46 it is possible to alkylate polyaniline base directly to
poly(N-alkylaniline) by using NaH in DMSO and the appropriate alkyl bromide.
[Edited on 4/1/2010 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
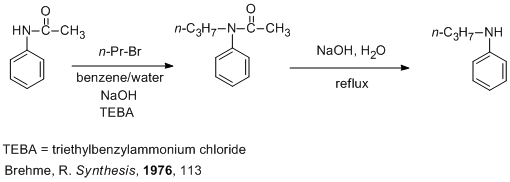
Jor, I think a way of obtaining N-alkylanilines which might work is the alkylation of acetanilide with n-alkylhalides (see the scheme and the attached
refernces) followed by hydrolisis with aqueous base. This alkylation procedes best using phase-transfer catalysis conditions. One reference cites
triethylbenzylammonium chloride as catalyst, but I think you could use other quaternary ammonium salts such as cetylpyridinium bromide etc. You can
obtain in this way N-propylaniline or perhaps N-butylaniline but less likely N-ethylaniline because of the low boiling point of ethyl bromide. Anyway
poly(N-propylaniline) is more soluble than poly(N-ethylaniline) in organic solvents. As Nicodem pointed out, it's very unlikely that
N-(isopropyl)aniline will polymerize at all.
As far as the sulfonation is concerned, I think you should consider oleum rather than chlorosulfonic acid. Chlorosulfonic acid is nasty stuff, fumes
like hell in air (HCl gas), the reaction with water is extremely exothermic.
If you want to acetylate polyaniline, you'll have to obtain poly(leucoemeraldine) first. As you said, phenylhydrazine or hydrazine hydrate are used as
reducing agents. There are many references as far as the reduction is concerned, I think it would be best to use hydrazine hydrate at room
temperature, though the reaction takes like 72 hours (Synthetic Metals, 1989, 29, E243). The reaction however must be done under inert atmosphere, and
all the subsequent steps like filtration of the polymer, washing with methanol and reacting with acetic anhydride must also be done under argon
because poly(leucoemeraldine) oxidizes in air very quickly.
Attachment: jor.zip (1.1MB)
This file has been downloaded 646 times
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Thanks, I didn't think of that way to produce mono N-alkylated anilines.
The inert atmosphere is rather problematic for me. Yes I can generate some N2 or CO2 (CO2 form bicarbonate, N2 from ammonium chloride and sodium
nitrite), but i cannot have a continuous flow of the gasses, i don't have the cylinder, and they are extremely expensive. Stoppering the reaction
mixture seems dangerous, as N2 gas is formed when reducing with hydrazine. Do you know of alternative reducing agents? I have many at hand, like
hypophosphite, zinc powder, tin(II)chloride, dithionite, etc (although i rather don't use hypophosphite as it's hard to get).
Filtering in the absence of air is easy, I will just cover the filter with a watch glass and fill the filting flask with inert gas. The acetylating
step is also easy, just stopper the flask, as there is no gas evolution.
What do you mean with oxidising quickly? Is it like chromium(II) salts, so instantly?
Why is chlorosulfonic acid more hazardous than oleum? Oleum gives of extremely dense H2SO4 fumes. And instantly chars skin.
By the way wiki states that leucoemeraldine is poor conducting of electricity so aren't leucoemeraldine derivatives as well? Can't I acetylate the
emeraldine straight away?
[Edited on 5-1-2010 by Jor]
|
|
|
matei
Hazard to Others
  
Posts: 205
Registered: 16-9-2006
Member Is Offline
Mood: No Mood
|
|
Well, you can use other reducing agents such as sodium dithionite and sodium borohidride (see Makromol. Chem. 1993, 194, 3149).
The oxidation of poly(leucoemeraldine) isn't instantaneous, actually it takes several days of oxygen exposure to obtain emeraldine base. (Synth. Met.
1997, 89, 63, J. Chem. Soc., 1910, 2388).
Attachment: Chemical reduction of the emeraldine base of polyaniline by reducing agents and its kinetic study.pdf (341kB)
This file has been downloaded 1323 times
Leucoemeraldine, emeraldine base and pernigraniline are insulators. Emeraldine salt (such as emeraldine hydrochloride) is the only form of
polyaniline that's a conductor, but all the transformations between the polyaniline forms are reversible, so theoretically you can switch from
poly(leucoemeraldine) to poly(emeraldine HCl) by oxidation and acid treatment.
[Edited on 5-1-2010 by matei]
Attachment: Aniline-black and allied compounds. Part I.pdf (1.1MB)
This file has been downloaded 851 times
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
The oxidation of aniline to produce a black (and therefore likely to be electrically conducting) polymeric substance has been known for a very long
time. It was discovered by William H Perkin (later Sir, or Cur if you like), at the age of 18, in 1856, when a student at the Royal College Of
Chemistry in London. He oxidized aniline (prepared by nitration of benzene, which however had some toluene in it, followed by reduction with nascent
H2 or catalytically with H2 and Pt or Pd) with K2Cr2O7 in acid solution to obtain a tarry black product, which was chiefly polyaniline. It is only
recently, however, that its use as an electrically conducting polymer, due to its infinitely conjugated double bonds, has been exploited.
Perkin, by careful extraction of it with CH3OH, isolated a few percent of a beautiful purple pigment which had permanent-dye properties, which he
called "mauve", and which created a sensation, being the very first synthetic dye (with the possible exception of picric acid and trinitroluene, also
obtained by nitration, used as yellow dyes before their use as explosives was discovered). Its molecule, a polycyclic quaternary ammonium salt,
contains 4 N atoms, one each a primary aromatic amine, secondary aromatic amine, tertiary amine in an aromatic ring, and quaternary ammonium salt in
an aromatic ring. Perkin resigned as a student, and coming from a wealthy family, formed a company to exploit his discovery by manufacturing the stuff
(entailing production of large amounts of aniline from benzene) at a time when there was little real chemical industry in England. He made an huge
fortune from the stuff, although his discoveries were later superseded by other dyestuffs (e.g. azo dyes) from the late 1860s.
Subsequent analysis found that Perkin's mauve was not, in fact, derived from pure aniline, but from the small amount of methylaniline (mostly ortho
isomer, with some para) impurity in it, resulting from the original benzene used for nitration containing some toluene. Pure aniline yields a similar
dye, however, without two methyl groups on the perimeter of the mauve molecule, although less readily than methylaniline, and this came to be marketed
under the name pseudomauveine, a less popular red-brown dye which was used in the "penny-red" British postage stamps.
See: http://en.wikipedia.org/wiki/Polyaniline , http://en.wikipedia.org/wiki/Mauve , http://en.wikipedia.org/wiki/Mauveine , http://www.cavemanchemistry.com/cavebook/chtar3.html
[Edited on 5-1-10 by JohnWW]
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
I finaaly had a little time to continue on this project.
About 0,6g of previously prepared polyaniline (emeraldine form) was reduced by magnetically stirring it in 20mL 2M ammonia and adding a big excess of
hydrazine (about 1,5mL 55% N2H5OH, or 35% N2H4), followed by heating to about 60-70C, and covering the erlenmeyer with a watchglass . There was
constant gas evolution (wich is probably N2, as NH3 does not boil out at such concentration/temperatures). When the gas evolution ceased, the
suspension was boiled for a few seconds to make the precitipate somewhat courser, and it was gravity filtered (I don't have vaccuum.
Because the filtering was slow, the solid somewhat air sensitive (although it is not oxidised in minutes, I read hours/days), and water would not be
ideal in the following reaction because it reacts with Ac2O, the precitipate was washed with EtOH, followed by ether (as EtOH also reacts with Ac2O).
Then when the solid in the filter was dry, by blowing some air over it, it was transferred to a beaker and about 4-5mL acetic anhydride was added and
the beaker was covered. The suspension was heated to 80C. After 2 hours there is a dark red suspension.
It seems the acetic anhydride dissolved quite some of the N-acetylated polyaniline, because when a a few drops are added to hot water, the acetic
anhydride quickly reacts and dissolved and a precitipate is formed.
I will destroy remaining the acetic anhydride tomorrow with excess water, and test the solubility of the polymer in some solvents like acetone,
methanol and chloroform.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
I added about 20mL of water to the acetic anhydride while hot, and it was almost instantly destroyed. There was a dark purple suspension, wich I
filtered.
The wet precitpate was put in a beaker and the water was evaporated. A black solid was obtained. About 300mg of it were scraped in a vial, and the
rest was stuck to the bottom of the beaker.
So I tested a few solvents. A few mL MeOH dissolved almost nothing, but boiling MeOH dissolved at least some of the polymer, giving a dark purple
solution. The methanol was boiled away.
Chloroform (2-3mL), even when cold, gave an intense purple solution.
Acetone even when hot, only gives a light purple solution, it is not effective in dissolving the polymer.
It is hard to determine how much polymer dissolved, but it is clear that chloroform (and probably dichloromethane) are relatively good solvents for
N-acetylated polyaniline.
I'm not sure why the aqeous phase dissolved so much of the polymer (solution is dark purple). I think water on it's own is a worse solvent for the
polymer than water, considering the structure of the polymer. So the cause is probably acetic acid, the hydrolysis product of acetic anhydride, that
protonates NH-groups wich are not acetylated?
I will try more experiments when I have time, but I think this is not very soon.
|
|
|
|