ChrisWhewell
Hazard to Self
 
Posts: 66
Registered: 22-12-2009
Location: Austin
Member Is Offline
Mood: No Mood
|
|
Bismuth - Tellurium
If I mix bismuth metal powder and tellurium powder and heat, should I expect them to alloy, or should I expect a violent reaction ?
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I expect them to react, forming bismuth telluride (Bi2Te3), but certainly there can be a non-stoichiometric reaction in which BixTey is formed (x, y)
different from (2,3). Be careful with tellurium, especially when it is heated. Tellurium gives rise to horrible smell when it is metabolized in the
human body. You run the risk of being very smelly for many days or even weeks when you inhale tellurium dust or vapor. If tellurides are formed, then
you also run the risk of inhaling H2Te.
|
|
|
Mr. Wizard
International Hazard
    
Posts: 1042
Registered: 30-3-2003
Member Is Offline
Mood: No Mood
|
|
From what I've heard about Tellurium you will get a nasty stench breath, that won't go away for a while.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by woelen  | | I expect them to react, forming bismuth telluride (Bi2Te3), but certainly there can be a non-stoichiometric reaction in which BixTey is formed (x, y)
different from (2,3). Be careful with tellurium, especially when it is heated. Tellurium gives rise to horrible smell when it is metabolized in the
human body. You run the risk of being very smelly for many days or even weeks when you inhale tellurium dust or vapor. If tellurides are formed, then
you also run the risk of inhaling H2Te. |
I would expect Bi and Te to form a series of fairly low-melting
alloys throughout the composition range, including an eutectic, and probably with definite intermetallic compounds at certain compositions. From a
Google search, Bi2Te3 seems to be the intermetallic compound of greatest interest and importance, especially for making nanowires, see for example http://cfse.ps.uci.edu/Penner_nanoletters_2004.pdf and http://pubs.acs.org/doi/abs/10.1021/nl048627t . The Bi-Te binary phase diagram is among the ASM Alloy Phase Diagrams, but it does not seem to be
available as a free download; however, it should be in the ASM Handbook of which I have a PDF copy.
As for Te giving rise to an horrible smell (like garlic but worse) for a long time if ingested and metabolized in the body, due to the formation of
H2Te and (CH3)2Te (which are also very poisonous) - see http://en.wikipedia.org/wiki/Tellurium , the same would be expected if Po is ingested due to the formation of H2Po and (CH3)2Po, but even more
toxic because of the intense alpha-radioactivity of Po. I wonder if Col. Litvinenko, that expatriate KGB agent who was killed 3 years ago in London as
the result of a Po compound being added to his pot of tea in a café by another KGB agent, Lugovoi, who visited from Russia, was noticeably smelly in
the days he was in hospital before dying. However, this is not stated in http://en.wikipedia.org/wiki/Alexander_Litvinenko_poisoning .
[Edited on 23-12-09 by JohnWW]
|
|
|
ChrisWhewell
Hazard to Self
 
Posts: 66
Registered: 22-12-2009
Location: Austin
Member Is Offline
Mood: No Mood
|
|
I think before one gets any H2Te in there, they'd need to get some H in there and also a reduction of the Te. Heating Bi with Te in the absence of
any H probably won't allow that to form. My guess as to what would happen is that the Bi would all agglomerate into a ball and by surface tension
not permit any of the Te to contact it sufficiently intimately for reaction, otherwise I could find something in the literature on this. Just a guess
though.
Imagine all the old prospectors of the old west in the gold rush days who roasted ores that typically contained tellurides and what they breathed !!
I once read where the prospectors would amalgamate their gold to separate it from the gangue to get a nice gold-amalgam and the way they separated
the gold was by slicing a potato in half, and hollowing out each half with a spoon to leave a cavity. They'd then put the Au-amalgam in the cavity,
put the other half of the potato onto the first half and wire it together, then bake it in the camp fire. This caused the Hg to be absorbed into the
potato, leaving behind a pure gold button upon opening. One account I read recommended burying the potato so animals wouldn't eat it. I cant help
but wonder about the Hg levels in the sierra-nevada.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
This paper does not make mention of Bi-Te alloys as being energetic, and the authors were looking for energetic intermetallic reactions. I would not
really be worried.
http://www.osti.gov/bridge/purl.cover.jsp;jsessionid=FEFEBE6...
I have linked to the bismuth-tellurium phase diagram. I was going to ms-paint out my toes, but once I did one, I wondered why I was bothering...
The diagram will tell you what compounds can be formed, among other information.
I uploaded it to scipics as opposed to as an attachment as I did not want to loose image quality.
http://www.sciencemadness.org/scipics/BiTe.jpg
[Edited on 23-12-09 by The_Davster]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Mercury was also used to amalgamate fine gold picked up by dredges in mountain streams in the West. I had a miner tell me that the streams around his
claim in Idaho contained a lot of mercury. I imagine it was losses from those dredges.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by ChrisWhewell  | | I think before one gets any H2Te in there, they'd need to get some H in there and also a reduction of the Te. Heating Bi with Te in the absence of
any H probably won't allow that to form. |
Not immediately, but as there always is humidity in the air, you
certainly will end up making some H2Te if indeed you make tellurides. Think of sulfides, they always smell of rotten eggs, simply due to reaction with
water vapor from the air. Tellurides will be more sensitive to this reaction.
|
|
|
Pyrovus
Hazard to Others
  
Posts: 241
Registered: 13-10-2003
Location: Australia, now with 25% faster carrier pigeons
Member Is Offline
Mood: heretical
|
|
Quote: Originally posted by JohnWW  |
As for Te giving rise to an horrible smell (like garlic but worse) for a long time if ingested and metabolized in the body, due to the formation of
H2Te (which is also deadly poisonous) and (CH3)2Te - see http://en.wikipedia.org/wiki/Tellurium , the same would be expected if Po is ingested due to the formation of H2Po, but even more toxic because of
the intense alpha-radioactivity of Po. I wonder if Col. Litvinenko, that expatriate KGB agent who was killed 3 years ago in London as the result of a
Po compound being added to his pot of tea in a café by another KGB agent, Lugovoi, who visited from Russia, was noticeably smelly in the days he was
in hospital before dying.
[Edited on 23-12-09 by JohnWW] |
That would seem unlikely. Given how little Po-210 is required to kill someone, I'd imagine that if there were ever assembled enough (CH3)2Po to
actually be able to smell it, it would also be enough to kill the smeller, many times over.
[Edited on 24-12-2009 by Pyrovus]
Never accept that which can be changed.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
While you can make bismuth telluride by heating the elements, I notice that most papers start with mixtures of the oxides or similar compounds and the
reduce them. While this would seem to offer the hazard of producing TeH2, there must be some reason they picked those routes over mixing the free
elements. In some cases they were doing vapour phase depositing of thin films, and their alternative route is sensible. But in others they were after
the bulk product, and the reasoning is less clear. US patent 5458867 is an example.
Electrochemical depositing of films is also done, for example http://pubs.acs.org/doi/abs/10.1021/cm060171o http://www3.interscience.wiley.com/journal/119814809/abstrac... while there's some detail in the freely accessible PDF http://www.usc.edu/CSSF/History/2005/Projects/S0508.pdf
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Bi-Te binary phase diagram
I have now had an opportunity to search the ASM Metals Handbook, Vol.3 (PDF), for the Bi-Te phase diagram. Here it is.
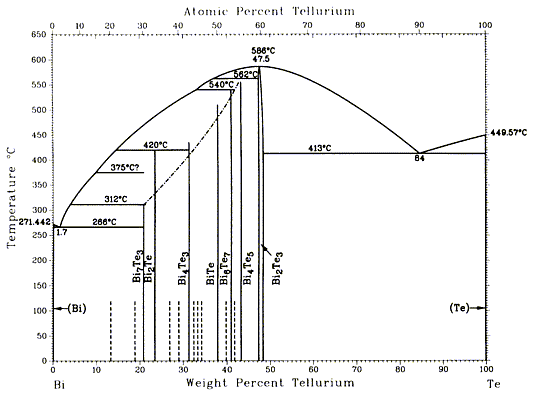
|
|
|
ChrisWhewell
Hazard to Self
 
Posts: 66
Registered: 22-12-2009
Location: Austin
Member Is Offline
Mood: No Mood
|
|
thanks alot for that  I couldn't find it. I couldn't find it.
|
|
|
ChrisWhewell
Hazard to Self
 
Posts: 66
Registered: 22-12-2009
Location: Austin
Member Is Offline
Mood: No Mood
|
|
Those were my thoughts as well. I had seen the ED method. I also saw a patent using a solution technique, US Patent 5,458,867. 6043424 is of
interest. The solution techniques generate waste that I want to avoid generating. I need about 100g of pure BiTe for a prototype unit.
|
|
|