| Pages:
1
2 |
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
neodymium sulfate??
I have purchased some 99.9% Nd2O3. When I add this to 20% H2SO4 and heat somewhat, then it dissolves fairly easily, giving a brown/yellow liquid (when
viewed under white TL light) or a pink liquid (when viewed under tungsten light).
If I continue heating, until the liquid starts boiling, then a crystalline solid is formed (neodymium sulfate only is very sparingly soluble in
boiling water, while it dissolves quite well in cold water). The solid is pink. The liquid above the solid is yellow/green.
When the crystals are taken away and rinsed with very hot water and then dissolved in cold water, then a pale lavender solution is obtained (when
viewed under TL-light). The yellow/green liquid remains yellow/green when it cools down.
Finally, I added some sulphuric acid to the lavender solution and then it remains lavender.
So, now I have two solutions of neodymium sulfate in dilute sulphuric acid. One of them is purple/lavender, the other is yellow/green. Can someone
explain the difference to me?
|
|
|
merrlin
Hazard to Others
  
Posts: 110
Registered: 3-4-2009
Member Is Offline
Mood: No Mood
|
|
I can't explain it, but I am wondering if the oxygen released during dissolution of the oxide might have played a role. Was gas evolution observed
during dissolution?
|
|
|
kmno4
International Hazard
    
Posts: 1523
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
My Nd2(SO4)3x8H2O has nice pinky colour in solid state or in solution ( something like diluted Co(II) solution) under tungsten lamp.
Under TL light solid or solution is almost colourless - I did not know about it until now.
During preparation of my sulfate I did not notice any yellow/brown colour.
Your Nd2O3 seems to be contaminated by 'something'.
What is its colour ?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Yellow-green sounds like praseodymium to me, although its solubilities are very similar to neodymium so a separation would seem unlikely. Note that
'didynium' salts were common in the earlier 20th century, being the remaining rare earths after the separation of cerium. The didynium salts colour
varied from the pinkish when Nd predominated to brownish as the amount of Pr increased.
The old book The absorption spectra of solutions of certain salts of cobalt, nickel, copper, iron, chromium, neodymium, praseodymium, and erbium
in water, methyl alcohol, ethyl alcohol, and acetone, and in mixtures of water with the other solvents, by Harry Clary Jones & John Augustus
Anderson is available on-line for download, and might be of interest.
[Edited on 14-10-2009 by not_important]
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
See http://www.sciencemadness.org/talk/viewthread.php?tid=8758&a...
Neodymium salts are usually violet to lavender, see the oxalate produced by kmno4 shown in that thread. I produced my sample of sulphate via the
chloride, which is violet in solution. The German thread mentioned by me produced it by direct action with sulphuric acid - their result was violet.
Like kmno4, the stuff I have is pinkish red. CRC says this is the color of the unhydrated salt, but usually all RE metals sulphates produce hexa to
octohydrates (maybe this is an error - perhaps The Davster knows, he seems to be well conversed with the RE metals).
Either your result is due to a differing level of hydration or there is still a lot of H2SO4 present in solution, is my guess. I haven't tried to
dissolve my sample in strong H2SO4, but that could change the hydration level. Or maybe there is some association of acid and Nd+++ ions.
A yellowish sample of cerium sulphate I produced refused to become white on several recrystallizations. I gave up once I discovered, on microscopic
examination, a few reddish needles mixed in with white. There was obvious some other RE metal present, Probably Pr or Nd.
Regards, Der Alte
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
If oxygen was released on the dissolution of Nd2O3 in a non-oxidizing acid, that could only be due to contamination with a rare-earth metal in the
(IV) oxidation state, which only occurs for the earliest metals in the rare-earth series (particularly CeO2, and non-stoichiometric Pr6O11), and those
just after the middle of the series (particularly non-stoichiometric Tb4O7), due to 0 and 7 unpaired 4f electrons being preferred occupancies of the
seven 4f orbitals. Of these elements, Ce(IV) is the only one that can exist as (IV) salts, e.g. the nitrate, in aqueous solution. Nd, which follows
Pr, just might form a non-stoichiometric (IV) oxide under extreme conditions.
Any differences in color of preparations of Nd(III) sulfate could be due either to contamination by other rare-earth elements especially Pr, or to
different complexing ligands being present.
|
|
|
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I doubt that there is a contamination with Pr. If I dissolve the Nd2O3 in dilute HClO4, then I obtain a pink/lavender solution, no yellow color at
all. If I dissolve it in dilute HNO3 then I also obtain a lavender solution.
If the color were due to Pr-contamination, then I also would have the effect with HClO4 and HNO3. I also think that it has to do with some
coordination effect.
I know of another salt which shows such behavior. If you dissolve chrome alum or chromium(III)sulfate in water, then you obtain a greyish/purple
solution. If you heat such a solution, then you get a green solution. On cooling down it remains green. Could it be that the dissolving of the Nd2O3
in dilute H2SO4 leads to formation of some sulfato-complex which cannot be formed from Nd(3+) ions, which are hydrated?
I tried an other thing. If I add excess ammonia to the purple/lavender solution, I obtain a very light blue precipitate. If I add excess ammonia to
the yellow solution, I also obtain a very light blue precipitate. A precipitate of praseodymium hydroxide is pale green.
|
|
|
kmno4
International Hazard
    
Posts: 1523
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
What colour is your Nd2O3 ?
This old paper did not mention any mysterious colour but lavenda.
[Edited on 14-10-2009 by kmno4]
|
|
|
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
My Nd2O3 is light grey with a faint bluish hue. Here you can see a picture. The blue hue is very hard to capture with the camera, it simply looks
light grey.

[Edited on 14-10-09 by woelen]
|
|
|
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The solution of the neodymium solutions also depends strongly on the light used. The top picture shows solutions of the sulphate, nitrate and
chloride, all in excess acid in daylight. The bottom picture shows the same ampoules, but now under fluorescent light.


It is remarkable that both the sulfate and chloride show the yellow color under the FL-light, but the nitrate appears colorless under the FL-light.
When I take the pink crystals of the sulfate and I dissolve these in some dilute sulphuric acid, then the resulting solution is like the nitrate
solution, even after boiling some time and cooling down again.
|
|
|
kmno4
International Hazard
    
Posts: 1523
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Hm...
I would try to rule out Fe(III) contamination by adding some thiocyanate. As far as I know Nd does not give any strongly coloured complexes with
SCN(-).
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
If you have a UV source, try under that. The RE ions are known for fluorescence, but I don't know what wavelengths work.
Der Alte
|
|
|
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Quote: Originally posted by kmno4  | Hm...
I would try to rule out Fe(III) contamination by adding some thiocyanate. As far as I know Nd does not give any strongly coloured complexes with
SCN(-). |
I tried this already. This also came into my mind as one of the first things. There is no strong reaction with thiocyanate.
The UV test is something I can do this evening. I have a UV-C source (254 nm), a blacklight source (around 365 nm) and a UV LED (around 390 nm).
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
It would be interesting to split the yellow-green solution in half, maybe in a larger scale repeat of the original experiment. Concentrate one half to
see if you can get it to form crystals, if it doesn't cooperate then add alcohol or acetone to try to force it from solution. Add ammonia to the
second portion to get the pale lavender ppt, wash it, and the repeat the dissolution in 20% H2SO4 to see what happens. Similarly take some of the pink
crystals formed from boiling, dissolve them in cold water, add ammonia to get the hydroxide, dissolve in H2SO4 to see if heating causes a similar
'splitting' into pink solid and yellow-green solution.
I can't find a description of two forms of Nd(III) sulfate, but my books are fairly old - most back when getting pure REE compounds was still
difficult. The brown-yellow colour of Nd(III) sulfate in concentrated solution with excess H2SO4 is described, it does appear that sole sort of
sulfate complex is formed.
On the other hand, Nd(III) tends to swamp the colour of Pr(III) , as Nd has several strong bands in the 500..600 nm range where the eye is most
sensitive; Pr(III) has narrower bands there with most of its wider stronger ones below 480 nm. A mixed hydroxide might look more like Nd(OH)3. And
generally the absorption bands of the REE aren't strongly affected by their environment, unlike the d-block elements.
A proper absorption spectrum would be enlightening, I suspect.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by JohnWW  | | If oxygen was released on the dissolution of Nd2O3 in a non-oxidizing acid, that could only be due to contamination with a rare-earth metal in the
(IV) oxidation state, which only occurs for the earliest metals in the rare-earth series (particularly CeO2, and non-stoichiometric Pr6O11), and those
just after the middle of the series (particularly non-stoichiometric Tb4O7), due to 0 and 7 unpaired 4f electrons being preferred occupancies of the
seven 4f orbitals. Of these elements, Ce(IV) is the only one that can exist as (IV) salts, e.g. the nitrate, in aqueous solution. Nd, which follows
Pr, just might form a non-stoichiometric (IV) oxide under extreme conditions. (cut) |
P.S. I have done some checking, and Nd itself does not form any (IV) oxide or fluoride, although the possibility of it probably has been examined
using atomic oxygen and fluorine gas; see http://en.wikipedia.org/wiki/Neodymium . So any liberation of oxygen on dissolution of Nd2O3 in acid is almost certainly due to contamination with
Pr6O11, which is formed on combustion of Pr in air (PrO2 can be obtained with atomic oxygen, and PrF4 with fluorine), and which instantly liberates
oxygen on contact with aqueous acids. Pr(III) salts, with two unpaired 4f electrons, are yellow-green. The possibility of Pr(V) oxide or fluoride,
with no unpaired electrons like Ce(IV) in CeO2 and CeF4 and ceric salts of oxy- and fluoro-anions, has probably also been examined, but found to not
exist.
Nd(III) has three unpaired 4f electrons, transitions of which at energies corresponding to visible light wavelengths must be responsible for the pale
blue or lavender or violet colors of its compounds. Since neodymium coloration depends upon "forbidden" 4f-4f transitions deep within the atom, there
is relatively little influence on the color from the chemical environment. That they are "forbidden" transitions, quantum-mechanically, accounts for
the paleness of most of its compounds, similarly to that of salts in low oxidation states of d-series transition metals such as Fe(II) and Mn(II).
I have uploaded the book Lanthanide_And_Actinide_Chemistry-0470010053_Wiley-2006_.rar , 2,277 Kb, to my Rapidshare Premium account. The link for it
is in the Inorganic Chemistry Books section in References.
[Edited on 15-10-09 by JohnWW]
|
|
|
kmno4
International Hazard
    
Posts: 1523
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
This thread froced me finally to recrystallize my Nd sulfate.
Clear, saturated solution (~28g Nd2(SO4)3x8H2O in ~300 ml H2O + few drops of H2SO4) is now set for evaporating.
Colour of this solution is pale pink undr tungsten light, but turns to very pale green under fluorescent lamp. This green colour is rather weak and in
thin layer of solution it looks like colourless.
Unfortunately my idioten-camera sees this green as no coloured solution 
I recalled experiment with solutions of Ni(II) and Co(II).
Almost colourless solution may be obtained when these solutions are mixed together in definite propotions.
This may link somehow with Nd(III) behaviour.
It is possible that Nd(III) solvates possess absorption bands similar to Ni+Co couple, of course in large approximation.
Resultant colour depends on these bands and kind of light.
BTW.
A books series about rare earths:
Handbook on the Physics and Chemistry of Rare Earths
is available as PDF files. Not very interesting but something to read about Nd.... etc.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kmno4  | (cut) A books series about rare earths:
Handbook on the Physics and Chemistry of Rare Earths
is available as PDF files. Not very interesting but something to read about Nd.... etc. |
The following
web-pages, among others, appear to have links from which to download the series as something like 40 volumes of PDFs:
http://ebook30.com/science/chemistry/132/handbook-on-the-phy...
http://www.ellibs.com/book/9780444521439
http://avaxhome.ws/ebooks/rare_earths_handbook_2_to_4.html
http://www.ebook3000.com/Handbook-on-the-Physics-and-Chemist...
http://freebooksource.info/wp-trackback.php?p=69732
http://bbs.bio668.com/read.php?tid=46167
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
I done not think there is anything wrong with your Nd sulphate, kmno4. I wasted a few hours tonight Googling around and 99% of sources say it's pink.
Only one, a manufacturer, American Elements, hedged their bets and said it was 'light purple' but in their MSDS said pink!
I could detect no fluorescence in black light UV (long wavelength). If my memory were better I should not have made the suggestion, having worked - in
the dim and distant past - with Nd-YAG lasers. The emission spectra on which this device works is 1060 nm in the near IR.
However, the absorption spectra for Nd+++ (like most RE) consists of very narrow lines in the visual region, around 580nm in the yellow orange
boundary. Other very sharp bands are c, 750 nm and 355 nm, at the very edges of visual. These just ax out a very small part of the spectrum and hence
cause peculiar color effects that depend critically on the light source and the detector (in our case the eye). I think this is what we are seeing.
Most of a broad spectrum is left intact.
Also, Nd sulphate crystals are usually + 8H2O as suspected.
I won't give a list of references, just quote one or two:
| Quote: | Acid and Basic Sulphates of Neodymium and Praseodymium. By Camille Matignon (Compt. rend., 1902, 134, 657—660).—
The neodymium hydrogen sulphate, Nd(HS04)3 or Nd203,3S03,3H2S04, produced either by evaporating down a solution of the neodymium salt of a volatile
acid with excess of sulphuric acid or by dissolving the normal sulphate in the boiling reagent and allowing the solution to cool, crystallises in
long, silky, deliquescent, pink needles, which have an oblique extinction inclined at 12° to the principal axis. This
form, however, rapidly becomes hydrated and changes into an aggregate of small, anisotropic crystals ; finally, the normal salt with 8H20 is obtained
mixed with excess of dilute sulphuric acid. One hundred parts of boiling concentrated sulphuric
acid dissolve 1'30 parts of the acid salt. The basic neodymium sulphate, (NdO)2S04 or Nd203,S03, obtained by gently igniting the normal salt, is an
insoluble, amorphous, pink powder stable at 1000°.
From : The metals of the rare earths By James Frederick Spencer (1915)
Neodymium Sulphate, Nd2(SO4)3,8H2O, is obtained by crystallising a solution of the oxide in sulphuric acid.267 It forms red monoclinic prisms
isomorphous with the corresponding sulphates of erbium, yttrium, and praseodymium.6s7 It is moderately
soluble in water; 100 grams of water dissolve the following weights of neodymium sulphate, calculated as anhydrous salt:—
Temperature . . . . o° 16° 3o° 5o° 8o0 1oo°
Grams 9-5 7-1 5-1 3-6 27 2-25.
An acid sulphate, NdH3(SO4)3l is formed in light rose-coloured silky needles in the same way as the corresponding praseodymium compound.446-6ss-623 A
basic sulphate, (NdO)2 SO4, is obtained by heating the normal sulphate.633-a23
The double sulphate Nd2(SO4)3,Cs2SO4,3H2O is formed in lavender-coloured crystals by mixing solutions of the two sulphates and crystallising.6s9
|
I dissolved some of my Nd2(SO4)3 in water. A light brownish red solution. I added H2SO4, 60%, to give about 50% H2SO4. The color disappeared and the
solution became milky white, clearing to give what was at first a white precipitate but later a very pale lilac. However, my wife called it faintly
pink! The solution looked colorless in sunlight and in fluorescent lamp, but took on a slight lilac tinge in tungsten, as did the ppt.
The eye and brain play strange tricks with colors, as photographers are well aware! I suspect the unusually sharp absorption spectra of the RE metals
had a lot to do with it.
Incidentally, I also read that in the Nd case the absorption does not depend on the phase (gas, solution, etc) or the ligand (in solution); _ it is a
property of the atom, inner elctrons not outer. However, the characteristic color of Nd +++ is lilac. Sulphate (red) and iodide (green) are
exceptions, apparently.
Der Alte
|
|
|
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Good to read that this thread gives rise to so much discussion and experimenting 
I also tested for fluorescence and there is no fluorescence at all. I tried UV-light around 370 nm (a blacklight tube), I also tried UV-LED's
(centered at 395 nm) and I tried a UV-C source (centered at 254 nm). None of these lead to fluorescence.
I also did an experiment with my lavender solution of neodymium sulfate (the one half of the 'split off' material, the other half was set aside). I
added a slight excess of ammonia, such that there just was a smell of ammonia from the solution. This results in formation of a very pale
blue/lavender precipitate. I filtered it with paper having very fine pores, using a vacuum pump (even then, filtering is soooooo slow). I rinsed with
distilled water and filtered again. This rinsed precipiate was split in two parts.
One part was added to 15% H2SO4. It quickly dissolves giving a lavender solution under TL-light and a pink solution under tungsten light.
The other part was heated in a quartz tube, using a propane torch. This results in formation of a blue/grey powder, just somewhat darker than my
original sample I have from eBay. After cooling down I added the dry powder to 15% H2SO4. It dissolves much more slowly then the freshly prepared
precipitate, and dissolving requires some heating. But finally, after a few minutes, all of it has dissolved. And guess what? The solution is
yellowish grey under TL-light and yellow under FL-light and pink in daylight and tungsten light, just like the solution I obtained when dissolving
some of my sample from eBay. I unfortunately could not get crystals from this solution, it was too dilute (all processing lead to loss of material),
but I am quite convinced that the calcined oxide really leads to another solution than the hydroxide.
[Edited on 16-10-09 by woelen]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Sounds convincing, yet a number of texts state that Nd2(SO4)3 is made by dissolving Nd2O3 in H2SO4. An absorption spectrum would be enlightening, I
suspect.
It is possible that you are forming a sulfo-complex, which is not described in any detail save being yellow-brown in tint - at that time under
daylight, incandescent light, or gas lights, all continuous spectrum sources. Fluorescent lights are generally discontinuous, with not too much
overlap with the Nd lines, mostly in the blue-green and yellow-green regions. The spike around 460-470 nm in white LEDs lands in the weak &
narrow blue bands of Nd, the longer wavelength bands of Nd should result in a greenish-yellow to pink colour depending on the exact phosphor in the
LED.
http://en.wikipedia.org/wiki/File:Fluorescent_lighting_spect...
http://en.wikipedia.org/wiki/File:White_LED.png
the attached image is of fluoroberyl , fluorophosphate, and silicate gasses doped with Nd(III). It doesn't quite math the aqueous spectrum, but shows
how little impact the environment has on the f-shell bands.
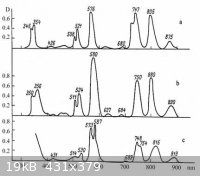
|
|
|
kmno4
International Hazard
    
Posts: 1523
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by not_important  | Sounds convincing, yet a number of texts state that Nd2(SO4)3 is made by dissolving Nd2O3 in H2SO4. An absorption spectrum would be enlightening, I
suspect.
|
Spectra of aquenous solutions of Ln are available in mentioned Handbook vol.3 in chapter 24.
Important feature of bands is also intensity and looking at pictures presented above I would not say that effect of matrix is small.
There is some effect in Ln solution: some absorption bands are very sensitive to chemical environmental (ligands) of Ln ions. It is called
"hypersensitivity" and may be responsible for different colours of sulfate and nitrate solutions.
BTW. I try to crystallize my sulfate because I know it is contaminated by Fe(III). It gives very weak but positive test with SCN(-).
[Edited on 17-10-2009 by kmno4]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
I meant a spectrum of woelen's yellowish liquid, to compare to reference ones.
I believe that the much of the colour change effects of ligands with lanthanides is more of changing the intensity of a line vs shifting its position,
unlike d-block elements where both intensity and peak can be greatly shifted.
|
|
|
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
The yellowish liquid is yellow in TL light, but brown with a somewhat pink hue in daylight. It is not the nice lavender/pink of other Nd-solutions at
all. Unfortunately I have no possibility of determining a spectrum of the liquid.
If someone of you has Nd2O3 of known high purity, then it would be nice to try what I did. Just dissolve some in a small amount of 15...20% H2SO4 and
boil out the neodymiumsulfate. It would be good to know what other's experience is with this simple experiment.
|
|
|
woelen
Super Administrator
        
Posts: 8149
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I now did a lot of further experimenting at a larger scale.
I took 5.0 grams of Nd2O3, 3.0 ml of conc. reagent grade H2SO4 and some water. I added the acid to water, such that I had appr. 40 ml of liquid. To
this, I added all of the Nd2O3. The Nd2O3 dissolves, giving a brown/yellow solution. Quite some heating is required to get all of it dissolved. When
all has dissolved, heating is continued, until boiling. This results in formation of a lot of pink crystalline precipitate and formation of a yellow
liquid above the precipitate (yellow in TL-light, golden yellow/brown in daylight). This golden yellow liquid I call liquid Y.
I decanted the yellow liquid from the pink crystalline mass (which can be done easily without introducing any losses, the precipitate really consists
of fairly large crystals). I rinsed with 20 ml of boiling hot water. This rinse water becomes yellow as well, but after a few swirls it becomes more
like grey (probably some of the solid dissolved, leading to a mix color of grey). I rinsed a second time with 20 ml of boiling hot water. This second
rinse was pale lavender. No yellow material is present anymore.
Next, I put the pink crystal mass on a filter paper, which in turn was put on a few sheets of household tissue. Most of the water was absorbed by the
household tissue. The solid material then was scraped from the filter paper and put in a petri dish and placed in a warm dry place (40 C, dry air,
free of dust). Within a few hours the material was dry and a pink solid is obtained. The solid is not hygroscopic at all. Yield is 5.1 grams, which is
appr. 50% of all neodymium (assuming that neodymium sulfate is Nd2(SO4)3.8H2O). The losses due to rinsing probably are a few percents and there may be
some mechanical losses, but I'm quite sure that at least 40% of the Nd is in the yellow liquid, which is decanted from the pink crystals.
I dissolved some of the pink crystal mass in water (which takes a loooong time) and tested for iron with concentrated ammoniumthiocyanate solution.
This test is 100% negative. The addition of the thiocyanate does not lead to any visible change of color.
I also tested the yellow liquid for thiocyanate. This liquid does show a positive, the liquid becomes pink/rose when thiocyanate is added at high
concentration. This means there is some iron, but only a very small amount.
Next, I took appr. 0.5 grams of the neodymium sulfate crytals, dissolved in water and then precipitated with ammonia. The precipitate was filtered and
rinsed with water and next it was heated to a red glow in a quartz tube. This calcined material was added to 20% sulphuric acid and dissolved by
heating. This leads to a brown/yellow solution. On boiling, a lot of pink crystalline solid was formed and unfortunately, now I had too little water
in it. ALL of the liquid turned to a pink solid, it only was wetted somewhat with water between the crystals and that liquid had a brownish/yellow
color.
From the yellow liquid Y (see above) I also took some and made a precipitate with ammonia. When I next add ust enough sulphuric acid to this liquid,
then the precipitate slowly redissolves again, but the color now has changed. I looks lavender. After a while the liquid becomes opalescent again. It
seems that there is some hydrolysis. I added a few more drops of dilute H2SO4 and then the liquid became totally clear again. Now its color had
changed again. It was grey in TL-light and almost colorless in daylight (very pale grey).
From this I conclude that there indeed exists a yellow/golden form of neodymium sulfate in solution and a lavender form. The difference must be the
coordination of sulfate to neodymium. There is some iron in the original Nd2O3 I purchased, but it is only a tiny amount. But it is there, so this
might affect the outcome somewhat.
Pictures of all the things, described above, will follow later this week.
[Edited on 20-10-09 by woelen]
|
|
|
kmno4
International Hazard
    
Posts: 1523
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
It is interesting.
I also dissolved Nd2O3 in H2SO4 without strange colours.
My eyes noticed only the pinks violets...
Also known to me literature does not say anything about yellow/brown colours in the case of simple salts of Nd.
Even in case of solid Nd(HSO4)3 or (NH4)[Nd(SO4)2(H2O)3]H2O only light-violet colour is reported (Acta Cryst. (2006). E62, i169–i172 and
Z. anorg. allg. Chem. 624 (1998) 1583-1587).
Unfortunately the X-MET I have access does not see this metal and Ln at all (I think). It shows mixture Mn, Fe, Zn... etc instead of Nd. 
If anyone knows any literature about yellow or brown colored simple salts of Nd, it would be very desired to post it here.
|
|
|
| Pages:
1
2 |