lordcookies24
Hazard to Self
 
Posts: 78
Registered: 2-1-2019
Location: pluto
Member Is Offline
Mood: curious
|
|
My half-assed electrolysis tank
I just made a electrolysis tank from a water bottle and pencils
[Edited on 7-1-2019 by lordcookies24]
 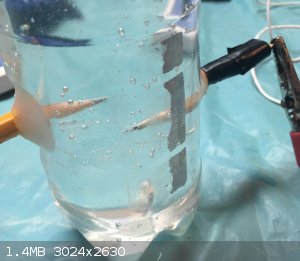
|
|
|
lordcookies24
Hazard to Self
 
Posts: 78
Registered: 2-1-2019
Location: pluto
Member Is Offline
Mood: curious
|
|
Graphite is a good electricity conductor so I used it as an electrode
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Are you making HHO gas with it?
The philosophy of one century is the common sense of the next.
|
|
|
lordcookies24
Hazard to Self
 
Posts: 78
Registered: 2-1-2019
Location: pluto
Member Is Offline
Mood: curious
|
|
If you mean hydrogen and oxygen then yes
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
just add brine then you can make chlorine and hydrogen
How I used to make my own HCl but that is tad more complex operation
[Edited on 7-1-2019 by XeonTheMGPony]
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
I hope that cap on top isn't pressurizing...
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
lordcookies24
Hazard to Self
 
Posts: 78
Registered: 2-1-2019
Location: pluto
Member Is Offline
Mood: curious
|
|
I actually used sodium bicarbonate as an electrolyte to avoid making chlorine
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
KOH is the normal one used for most commercial ones.
|
|
|
Sulaiman
International Hazard
    
Posts: 3730
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
have you collected hydrogen or oxygen (not the mixture) yet ?
made the hydrogen go POP, and re-light something with oxygen ?
(because I smoke I like to show a cigarette in (almost) pure oxygen,
some things cam burn VIOLENTLY so take care (or keep trying 
old school but still fun.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
lordcookies24
Hazard to Self
 
Posts: 78
Registered: 2-1-2019
Location: pluto
Member Is Offline
Mood: curious
|
|
But it’s definitely not the most available one. I can’t find potassium hydroxide in any store or pharmacies
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Go and check out supplement / health shops, I find it ironic the sheer number of chemicals you can find there, citric acid, gelitins, 35% peroxide
KOH, NOH, Glycerin
|
|
|
Sulaiman
International Hazard
    
Posts: 3730
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I think that sodium bicarbonate is a perfect choice;
. no chemophobia
. no strong caustic solutions
. no halogens
Commercially a highly conductive electrolyte is used for efficiency,
not the primary concern when using a 9V battery and tiny graphite electrodes..
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
KOH attacks PET bottles.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by unionised  | KOH attacks PET bottles.[/rquote
Does KOH solution eat PET bottles or are you talking about solid KOH attacking it? |
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
|
|
|
woelen
Super Administrator
        
Posts: 8030
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
With NaHCO3 the conductivity of the solution will be very low. NaHCO3 is not strongly soluble, you end up using quite dilute solutions and hence you
have low currents. Use of NaOH also is an option. NaOH is easier to find locally than KOH in most countries, as solid drain cleaner. NaOH is corrosive
and attacks PET, just as KOH does.
Use of dilute (e.g. 10% H2SO4) also works quite well and that does not attack PET as quickly as KOH/NaOH does.
Use of NaHCO3 has an added complication. At the anode you will get a mix of CO2 and O2. You will get acid as a result of electrolysis and this will
form CO2 with the NaHCO3:
Electrolysis will take away electrons at the anode. These electrons will come from water molecules:
2 H2O - 4e --> 4H(+) + O2
The H(+) then forms acid with remaining water and this in turns reacts with the HCO3(-) ion to form H2CO3 and hence H2O + CO2. Part of the CO2 will
dissolve in the solution, but if you try to get a high concentration of NaHCO3 (and you try, because otherwise your electrolysis is REALLY slow) then
CO2 is nearly insoluble and it escapes together with the O2.
You can alleviate the problem of formation of CO2 quite well by adding a little Na2CO3 to the solution as well. This makes the solution somewhat
alkaline and this makes CO2 much more soluble and may even prevent the formation of CO2 at all.
But in general, if you want O2 and H2, then it is best to use either:
- NaOH or KOH, you can even use copper anode in this case, but you need a glass bottle
- dilute H2SO4
- concentrated solution of NaHSO4 (this is sold in nearly every part of the world as so-called pH-minus for swimming pools).
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by XeonTheMGPony  |
Go and check out supplement / health shops, I find it ironic the sheer number of chemicals you can find there, citric acid, gelitins, 35% peroxide
KOH, NOH, Glycerin |
NOH? You mean NaOH or is 'N' supposed to be a variable?
Also I do hope you have a tube leading out of that bottle cap...
[Edited on 1-8-19 by Abromination]
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Abromination  | Quote: Originally posted by XeonTheMGPony  |
Go and check out supplement / health shops, I find it ironic the sheer number of chemicals you can find there, citric acid, gelitins, 35% peroxide
KOH, NOH, Glycerin |
NOH? You mean NaOH or is 'N' supposed to be a variable?
Also I do hope you have a tube leading out of that bottle cap...
[Edited on 1-8-19 by Abromination] |
Good catch
|
|
|