Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
Final reduction of alkali Nitrate/Nitrite
If you heat Sodium nitrate beyond decomposition to nitrite and 1/2O2, what is the final reduction (in absence of any additional reducing agent)?
Imagine it depends on the amount of heat applied. Also with sodium bicarbonate, for example, after the carbonate is formed, what sort of heat is
needed to turn it into sodium oxide? Kiln temperatures and beyond? Making the oxide in this way would be a good way of making very pure LYE OTC.
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
I had a NaNO3-melt not decomposing much even when it was glowing (in the 500s of [Celsius]), although in the books the _starting_ temp. for
decomposition is given to about 340 [Cels] ...
|
|
|
kmno4
International Hazard
    
Posts: 1497
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
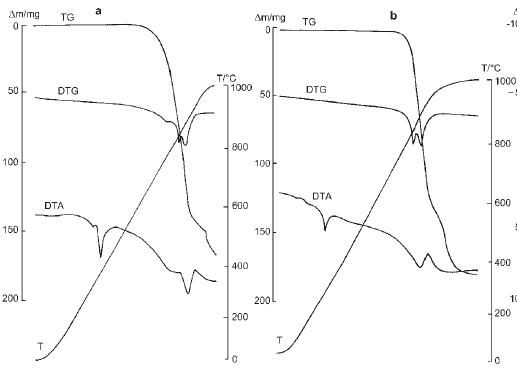
According to:
Journal of Thermal Analysis and Calorimetry, Vol. 60 (2000), p.305–-312,
thermal decomp. of NaNO3 starts at about ~700 C (air atmosphere).
Final product is Na2O, but this stage is obtained at temp. ~1000 C. Decomositin of NaNO2 is also described in this paper.
On the picture: NaNO3 (a) and NaNO2 (b) thermic curves.
|
|
|