Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
4-Aminobenzoic acid from Paracetamol
I am currently trying to prepare 4-Aminobenzoic acid.
There are a few simple ways to this, like nitration of toluene, followed by separation of the isomers, oxidation of the methyl group then reduction of
the nitro group.
This however is long, yields aren’t the best and some of us don’t have access to Toluene so that is out.
I have however thought of something else, starting from readily available, cheap Paracetamol.
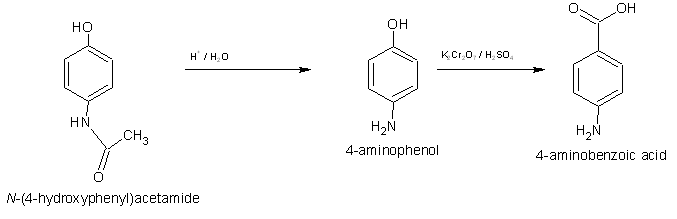
The synthesis involves this:
First acid catalyzed Hydrolysis of the ethyl group leaves 4-Aminophenol. The 4-Aminophenol can then be oxidized with dichromate/acid to 4-Aminobenzoic
acid.
There are a few problems with this however, mainly I have a feeling the NH2 group will be oxidized as well as the –OH group,
I am not a hundred percent certain, however I think if you oxidize the Paracetamol first, followed by hydrolyses this will leave 4-Aminobenzoic acid,
am I right?
Any comments/suggestions welcome,
Thanks,
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
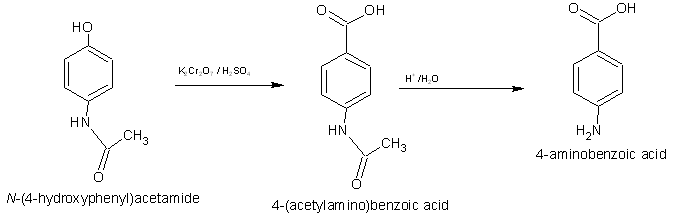
Sorry to double-post however i dont know how to add another image without deleting the last one 
This is the second method to 4-Aminobenzoic acid
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Magically growing carbons in that second step, are we?  Or first step if we look
at the second post. Or first step if we look
at the second post.
Maybe the half-amide of terephthalic acid can undergo a hofmann rearrangement to your desired product, but making the half-amide would be a pain in
the ass, unless you can make the half-ester to shield one of the two carboxylate groups.
[Edited on 1-28-09 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
I guess he confused the phenol and the benzylic alcohol! Your one carbon short I'm afraid!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Ah that is annoyin, i see that now, sorry im rather new to organics.
Is there any way to add one? Would you be able to methylate the -OH group follwed by oxidation with KMnO4?
It seemed too good to be true 
[Edited on 28-1-2009 by Picric-A]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Picric-A
...
Is there any way to add one? Would you be able to methylate the -OH group follwed by oxidation with KMnO4?
... |
That would be
Ar-O-H => Ar-O-CH3
the methyl is attached to the oxygen.
You could just purchase PABA.
If you really insist on making it, mono-nitration of polystyrene in solution in DCM or nitrobenzene will give mostly para substitution due to steric
effects. The nitrated polystryrene then can be oxidised to chop the chains between aromatic rings to give nitrobenzoic acid, mostly para, and benzoic
acid from unsubstituted styrene units. Purification can be done based on solubilities, relative acid strengths, the standard fractional
crystallisation, and so on. Reduction gives the amino acid.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Cool, Have you tried this? Are you sure the aromatic rings will cut evenly under oxidation, with say KMnO4?
Seems fairly simple, thanks
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Not the ring,but the backbone -CH2-CHAr-CH2-CHAr- ...
The benzylic hydrogen is easily attacked, which leads to oxidation of the backbone.
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Haha bless your magic carbon disappearing reappearing trick :p
The usual mixed acid approach to nitrating toluene only gives a p: o isomer ratio of 0.6 because of the industrial importance of p-nitrotoluene there
was/is much research out there into increasing this ratio. For example nitration of toluene with nitric acid and a aromatic sulphonic acid
(toluene-2,4-disulphonic acid on clay) increases the p: o to 1.60 with a 90% nitrated product yield(1)! Separation of isomers based on solubility
would be fairly easy(2):
2-nitrobenzoic acid:
Solubility in water 6.8g/L
1g/3mL ethanol - 1g/2.5mL acetone - 1g/220mL chloroform - 1g/2.5mL methanol - 1g/4.5mL ether
3-nitrobenzoic acid:
Solubility in water 2.4 g/L (15°C)
1g/3mL ethanol - 1g/2mL methanol - 1g/4mL ether - 1g/2.5mL acetone - 1g/18mL chloroform
4-nitrobenzoic:
Solubility in water insoluble
1g/110mL ethanol - 1g/45mL ether - 1g/12mL methanol - 1g/20mL acetone - 1g/150mL chloroform
So if you nitrate toluene and then oxidize the isomers you obtain nitrobenzoic acids which can be easily separated based on their solubilities in
different solvents due to the differences in the dipoles caused by the positioning of the groups on the ring. In addition do a TLC or similar on your
product will reveal contamination from other isomers quite easily and a mp too. Although for most purposes I'd imagine one recrystalisation would
suffice.
Toluene can be had easily "OTC" or is cheap enough to buy the technical product and redistil once.
Nitration of polystyrene can be achieved with a mixture of fuming nitric acid (100%) and sulphuric acid (97%) (V:V 3:1) cooled to -5*C, the
polystyrene can be added and stirred but if the temperature rises above 0*C oxidative degradation can occur(3).
I do not know for sure, but I would imagine oxidative clevage of polystyrene would require hot alkaline potassium permanganate and probably a lot of
reflux. You would have to remove MnO2 sludge with SO2 and then acidify (conc H2SO4) to release the carboxylic acids and extract into an organic
solvent.
Ref:
(1)Kameo, T. Manabe, O. Chemistry Letters from the Chem. Soc. Jap. 1972 pp 33-34
(2)Fisher Scientific Product Data
(3)C, GroBe-Rhode/H. G. Kicinski/A. Kettrup. Chromatographia, volume 26, number 1, 1988 pp 209-214
edit: removed accidental  from my post in the p: o ratios!!! from my post in the p: o ratios!!!
[Edited on 29-1-2009 by panziandi]
|
|
|
Siddy
Hazard to Self
 
Posts: 81
Registered: 8-10-2007
Member Is Offline
Mood: No Mood
|
|
You know PABA is OTC anyway right?
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Yea, however only in vitamin form form which it needs to be extracted form the other crap in there, and the vitamins alone are fairly expencive.
I have not found an OTC source of toluene yet, and as a result that is out,
I was going to give the nitration-oxidation of polystyrene a shot, however hearing about the conditions, it seems more difficult than i thought it
would be...
hmmm
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Perhaps nitration just with normal 70% nitric acid and sulphuric acid would work? I mean it works for nitrating toluene perhaps it will work with
polystyrene just with a lower yield? Also it is less likely to require being kept below 0*C. Also polystyrene has the advantage of being dirty cheap!
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Just over a year ago in a thread on TEMPO, Nicodem included an attackment on nitration polystyrene:
http://sciencemadness.org/talk/viewthread.php?tid=10568&...
The goal there was to avoid degradation of the chain, which is unimportant for this purpose because the next step is to degrade the chain. They tried
4 nitration methods, using m-nitrotoluene and standard mixed nitrating acids gave the best results; nitrobenzene works similarly as a solvent and DMC
should suffice.
Another PDF is
http://publications.drdo.gov.in/gsdl/collect/defences/index/...
Then there is US Patent 3978118, with usual handwaving
| Quote: | This invention relates to a process for converting styrene or polystyrene or combinations thereof, to nitrobenzoic acid, particularly
para-nitrobenzoic acid, by subjecting the styrene or polystyrene to nitration and then subjecting the nitratedaromatic compound to oxidation.
...
The nitrated aromatic compound obtained above is then subjected to conventional nitric acid oxidation to obtain the desired product predominating in
para-nitrobenzoic acid. If the nitration procedure defined above has been carried out in the presence of a dehydrating agent, as described, the
dehydrating agent is first removed from the nitrated product before oxidation, since the presence of the dehydrating agent during oxidation can
interfere with the smooth operation of the oxidation reaction. |
A interesting but not so easy to do way of oxidising the polystyrene is reported here http://sciencelinks.jp/j-east/article/200219/000020021902A04...
this has also been done with a solvent consisting of a mixture of mixed dichlorobenzenes and butyric acid; the dichlorobenzenes being prepared from
long refluxing of p-dichlorobenzene with catalytic amounts of AlCl3.
Just about any method that will oxidise toluene, ethylbenzene, or isopropylbenzene, will work; although an inert solvent may be required.
As note polystyrene is cheap to free, so really high yields aren't necessarily needed.
BUT
How about pretty pure looking PABA powder, 100 g for US$ 11.25, shipping, handling, and bribes extra?
http://www.smartbomb.com/le00106.html
|
|
|