Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Anthracene
A member recently asked me whether anthracene can be prepared from napthalene and I had no immediate answer. A few moments with my old pal Merck and
this thread is my answer.
Very pure anthracene can be prepared from synthetic anthraquinone. See citation.
Anthraquinone is prepared industrially from phthalic anhydride and benzene in presence of AlCl3 (Friedel-Crafts acylation).
Merck on phenanthrene
" Isomeric with anthracene. Occurs in coal tar. Isoln: Ostermayer, Fittig, Ber. 5, 933 (1872); Glaser, ibid. 982. Purification (from contaminating
carba zole and anthracene): Clar, Ber. 65, 852 (1932). Formation from toluene, bibenzil, 9-methylfluorene or stilbene by pas sage through red-hot
tube: Graebe, Ber. 7, 48 (1874); Ann. 167, 161 (1879); Ber. 37, 4145 (1904). Also from coumar one and benzene: Kraemer, Spilker, Ber. 23, 85 (1890).
Pschorr syn thesis from o- nitrobenzaldehyde and phenyl acetic acid: Ber. 29, 500 (1896). From diphenylethylene: Cook, Hewett, J. Chem. Soc. 1933,
1098. Diene synthesis from 1-vinylnaph thalene and maleic anhydride: Cohen, Warren, ibid. 1937, 1315. From o- phenylbenzoic acid: Schoumlnberg,
Warren, Chem. and Ind. (London) 58, 199 (1939). By irradiation of stilbene: Mallory et al., J. Am. Chem. Soc. 84, 4361 (1962). Synthesis by double
succinoylation of benzene: Rahman et al., J. Org. Chem. 28, 3571 (1963). Structure: Trotter, Acta Cryst. 16, 605 (1963).'
Phthalic anhydride can be obtained by oxidation of napthalene.
So the answer, at least superficially, is Yes, in two steps.
Napthalene -> phthalic anhydride
Phthalic anhydride + Benzene -> Anthracene
It will take a little more time to get the details, where the devil always hides.
"Obtained from coal tar: Dumas, Laurent, Ann. 5, 10 (1833); Laurent, Ann. 34, 287 (1840); Anderson, Ann. 122, 294 (1862); J. Chem. Soc. 15, 44 (1862);
Auerbach, Das Anthracen und seine Derivate (Braunschweig, 1880); Perkin, J. Soc. Arts 27, 572 (1879); Lunge, Coal Tar and Ammonia (1916); Barnett,
Anthracene and Anthraquinone (London, 1921); Nanson, Textile Colorist 48, 605, 678, 751 (1926); 49, 19, 246, 557, 593 (1927); Houben, Fischer, Das
Anthracen und die Anthrachinone (Leipzig, 1929); Borrmann, Der Teer (Leipzig, 1940); Schumann, Kokereiteer (Stuttgart, 1940). Extensive patent
literature on purification. Prepn of very pure anthracene from synthetic anthraquinone: Clar, Ber. 72, 1645 (1939). Review: E. Clar,
Polycyclic Hydrocarbons 2 vols. (Academic Press, New York, 1964)."
Purification of anthracene from coal-tar is apparently a matter of some technical difficulty.
Here's Merck on anthraquinone:
: Produced industrially from phthalic anydride and benzene in the presence of aluminum chloride by a Friedel-Crafts reaction: Klip stein, Ind. Eng.
Chem. 18, 1327 (1926). From anthra cene with vanadium pentoxide, sodium chlorate, glacial ace tic and sulfuric acids: Org. Syn. coll. vol. II, 554
(1943). Convenient lab procedure: L. F. Fieser, Organic Experi ments (Heath and Co., Boston, 1964) pp 195-200. Reviews: de Barry, Barnett, Anthracene
and Anthraquinone (London, 1921); Phillips, Chem. Rev. 6, 157 (1929); Houben, Fischer, Das Anthracen und die Anthrachinone (Leipzig, 1929); R. H.
Chung in Kirk-Othmer Encyclopedia of Chemical Technology, Vol. 2 (Wiley-Interscience, New York, 3rd ed., 1978) pp 700-707."
[Edited on 11-11-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
It fluoresces a nice blue, but back when I was working with anthracene, my real target was preparing triptycene (anthracene + a suitable benzyne
precursor like diazotized anthranilic acid). Fun.
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Sauron, I believe he mass U2U'd us, or at least U2U'd several members. I got the same question as well as one about what to do with trichloroethylene.
Industrially, the production of phthalic anhydride is done by catalytic oxidation of naphthalene with oxygen gas. I haven't done a thorough search,
but I get the feeling a lab scale version of this procedure would be highly impractical and cracking an aromatic ring open by oxidation is probably
not too easy to do. Phthalic acid and it's anhydride are large scale industrial chemicals and should be procurable without too much hassle. IIRC,
there have been some experiments on mass-extraction of plasticizer from shower curtains and subsequent hydrolysis to yield the free acid on this
forum. I seem to recall it was fairly cheap and straightforward.
[Edited on 11-11-08 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
yES, PHTHALIC ANHYDRIDE IS FILTHY CHEAP, AND YES, THE OXIDATION FROM NAPTHALENE MIGHT BE A MESS ON BENCH SCALE. tHAT IS WHAT i MEANT BY THE
SUPERFICIALITY REMARK AND THE DEVIL BEING IN THE DETAILS. Sorry about the all caps. I accidentally hit the caps lock and did not notice. No intention
to shout!
Here is the take from Ullmann's:
3. Production
Four processes are used in the industrial production of anthraquinone today:
1) Oxidation of anthracene with chromic acid
2) Vapor-phase oxidation of anthracene with air
3) Naphthalene process
4) Synthesis from phthalic anhydride and benzene
Anthraquinone is produced from anthracene, where this is available from coal tar, either by oxidation with chromic acid in 48 % sulfuric acid or by
oxidation with air in the vapor phase. The oxidation with chromic acid is competitive, provided that the chromium(III) sulfate lye formed can be
processed to tanning agents. Anthracene with purity of ² 94 % is required for both oxidation processes; crude anthracene from coal tar must be
purified by recrystallization.
About 85 % of world production today is based on the oxidation of anthracene. Since the mid-1970s, anthracene production has fallen continuously,
creating a supply shortage. Therefore, the complex naphthalene process is gaining in importance. There is an adequate supply of naphthalene in coal
tar. If necessary, additional naphthalene can be isolated from the residual oils of gasoline reforming, a process common in the United States.
In the new naphthalene processes developed in Japan by Kawasaki and in Europe by Bayer anthraquinone is synthesized in three steps: Naphthoquinone is
prepared by vapor-phase oxidation with air. Butadiene is added to this naphthoquinone in a Diels-Alder reaction. The tetrahydroanthraquinone formed is
oxydehydrogenated. In this naphthalene process a significant amount of phthalic anhydride byproduct is produced.
In the synthesis of anthraquinone from phthalic anhydride and benzene approximately 1.4 t of aluminum chloride and 4 – 6 t of sulfuric acid per ton
of anthraquinone are used. This procedure is used in areas where anthracene is not available in sufficient amounts from coal tar. It may become
important again, as a result of the shortage of anthracene. Phthalic anhydride has become available at low prices and in sufficient quantities
following the introduction of the economical oxidation of naphthalene or o-xylene with air.
In the early 1970s another interesting anthraquinone process was developed by BASF. Styrene is first dimerized to 1-methyl-3-phenylindane in the
presence of an acid catalyst, which is then converted to anthraquinone in the vapor phase by oxidation with air.
(Sections 3.1 and 3.2 are ommited as they are concerned with anthracene -> anthraquinone. -Sauron)
3.3. Naphthalene Process
The naphthalene process developed by Kawasaki Kasei Chemicals [9] consists of three steps. In the first step naphthalene is oxidized in the gas phase
with air on a vanadium pentoxide catalyst to produce the relatively unstable naphthoquinone (4). Large amounts of phthalic anhydride form at the same
time. Because most of the naphthalene reacts, recycling of unreacted naphthaline is not necessary [10][11][12][13].
.
The hot reaction mixture is precooled in a gas cooler and then quenched and washed with water in a tower. The naphthoquinone is obtained mixed with
phthalic acid anhydride (or phthalic acid) as a suspension in water. The naphthoquinone is extracted with an aromatic solvent, for example xylene or
tetrahydroanthraquinone, to separate it from the phthalic acid. The small residue of acid is carefully removed by washing with dilute alkali
[14][15][16].
In the second step the naphthoquinone is reacted with butadiene in a Diels-Alder reaction to give 1,4,4 a,10 a-tetrahydroanthraquinone (5). The
Diels-Alder product is separated from unreacted naphthoquinone by extraction with aqueous alkali, which converts the quinone into a water-soluble
alkali-metal salt. The organic phase, which contains naphthoquinone, is recycled to the first step [10] , [11] , [17].
In the third step the aqueous tetrahydroanthraquinone solution is reacted with air. The anthraquinone that forms is insoluble in aqueous alkali and
can be isolated by filtration. The aqueous alkali is recycled into the tetrahydroanthraquinone extraction step [18]. The anthraquinone can be purified
further by vacuum distillation [9].
Phthalic acid is isolated from the aqueous phthalic acid solution by crystallization and filtration. It is heated to convert it to phthalic acid
anhydride. The phthalic acid anhydride can be further purified by distillation.
Kawasaki Kasei Chemicals operates a plant based on the naphthalene process that produces 3000 t/a. The plant has been in operation since 1980.
In Bayer's variation of the naphthalene process, naphthalene is oxidized with a mixture of air and recycled reaction gas on a vanadium pentoxide
catalyst to give naphthoquinone, phthalic acid anhydride and unreacted naphthalene [19][20][21]. The products of the oxidation along with unreacted
naphthalene are quenched and condensed into a liquid reaction mixture, which is reacted without purification with butadiene [22] , [23]. The
Diels-Alder product mixture that contains tetrahydroanthraquinone is reacted with air. The tetrahydroanthraquinone is oxydehydrogenated to give
anthraquinone. The naphthalene is removed by distillation [23][24][25]. The two end products, anthraquinone and phthalic acid anhydride, are separated
by fractional distillation [26].
3.4. Synthesis from Phthalic Anhydride and Benzene
In the first step o-benzoylbenzoic acid (6) is prepared from phthalic anhydride and benzene by a Friedel-Crafts reaction. In the second step
the o-benzoylbenzoic acid is cyclized to anthraquinone by heating with concentrated sulfuric acid. The primary product of the Friedel-Crafts reaction
is an aluminum chloride complex of the o-benzoylbenzoic acid, which can harden easily to form a compact mass in the reaction vessel. Several methods
have been proposed to overcome this problem. Based on a process described by G. HELLER already in 1906, condensation is carried out in excess benzene
to give yields of more than 95 %. Patents issued to Klipstein & Sons and the I. G. Farbenindustrie from 1923 to 1927 describe a solvent-free
process carried out in ball mills. The problem posed by the reaction mixture's baking can be reduced by adding ethylene glycol or 1,4-butanediol,
which causes a reduction in the necessary excess benzene or makes a solvent-free process possible. For both processes batch processing is used even
today.
Phthalic anhydride, benzene, and aluminum chloride in a molar ratio of 1 : 1 : 2 are allowed to react below 45 °C in iron ball mills fitted
with hollow axles for the addition of benzene and the removal of the hydrogen chloride formed during the reaction, or in a vessel equipped with a
strong horizontal agitator, possibly propelled from the bottom. The reaction mass, which is heterogeneous at first, liquefies, then gradually becomes
viscous as hydrogen chloride is continuously lost, and eventually forms a powder. During the reaction there is extensive foaming, producing several
times the original volume, a fact that must be considered when the charge is measured out. The reaction is complete after 1 mol of hydrogen chloride
per mol phthalic anhydride has been evolved. The reaction product is placed into dilute acid. The precipitated o-benzoylbenzoic acid is separated,
washed, and dried, providing a yield of more than 95 %.
3.5. Styrene Process
This process was developed in pilot by BASF.
Styrene dimerizes in the presence of acid catalysts, such as sulfuric acid [27] , [28] , phosphoric acid, or boric acid [29] , yielding primarily
1,3-diphenyl-2-butene, which cyclizes further on the same catalyst to 1-methyl-3-phenylindane [6416-39-3]. The yields obtained in this step are 85 –
90 %.
1-Methyl-3-phenylindane is converted directly to anthraquinone by oxidation with air in the vapor phase [30].
Basically, the same catalysts are used as those employed in the gas-phase oxidation of anthracene with air, i.e., vanadium compounds, primarily
vanadium pentoxide in combination with other oxides, such as thallium oxide and antimony oxide [31].
Yields of » 77 % are obtained in the oxidation stage. Byproducts are still attached to the precipitated reaction product, and further purification is
necessary.
8. References
ï [9] Kawasaki Kasei Chemicals Ltd., Research and Development in Japan awarded the Okochi Memorial Price, 1980, Okochi Memorial Foundation.
ï [10] Kawasaki, JP 5108256, 1974.
ï [11] Kawasaki, JP 5108257, 1974.
ï [12] Kawasaki, JP 5322559, 1978.
ï [13] Kawasaki, DE 3033341, 1980.
ï [14] Kawasaki, JP 5251356, 1975.
ï [15] Kawasaki, GB 2039897, 1978.
ï [16] Kawasaki, JP 5422246, 1979.
ï [17] Kawasaki, US 4412954, 1981.
ï [18] Kawasaki, JP 5652434, 1981.
ï [19] Bayer, DE 2532422, 1975.
ï [20] Bayer, DE 2453232, 1974.
ï [21] Bayer, DE 2532365, 1975.
ï [22] Bayer, DE 2532388, 1975.
ï [23] Bayer, DE 2218316, 1972.
ï [24] Bayer, DE 2245555, 1972.
ï [25] Bayer, US 4284576, 1975.
ï [26] Bayer, DE 2532450, 1975.
ï [27] J. Risi, D. Gauvin, Can. J. Res. Sect. B. 14 (1936) 255.
ï [28] P. E. Spoerri, M. J. Rosen, J. Am. Chem. Soc. 72 (1950) 4918.
ï [29] BASF, DE 2064099, 1970.
ï [30] BASF, DE 1934063, 1969.
ï [31] BASF, DE 2135421, 1971.
ï
[Edited on 11-11-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
In the last post I emphasized in bold the phthalic acid -> o-benzoylbenzoic acid -> anthraquinone route simply because it appears to be the most
practical to do in a home lab.
So the problem devolves to:
a) Do we try to get to phthalic anhydride from napthalene? Or
b) Do we just buy it or prepare it from, say, o-xylene?
I seem to recall that "Fundamental Processes of Dye Chemistry" has prep of phthalic anhydride.
It is also a good time to look for bench scale preps of o-benzoylbenzoic acid.
And it is appropriate to see what is entailed in that Clar, Ber. 72 1645 (1939) reference from Merck for anthraquinone -> anthracene.
I would also like to know why the member who mass-PM'd the rest of us about this failed to take these simple and obvious steps himself?
Three pages from "Fundamental Processes of Dye Chemistry" detail the lab prep of phthalic anhydride by air oxidation of napthalene vapor over V2O5 on
pumice at 450 C. It is stated that 20 g can be obtained if the proper temperature and throughput is maintained and that if so the product is
chemically pure.
Several of us have commercial tube furnaces and several others have built their own and posted about it. 450 C is not an onerous temperature
requirement. V2O5 is not hard to get and pumice support is commonly available in a variety of sizes. Here 3mm is preferred. The rest of the apparatus
is well described and illustrated. So making phthalic anhydride in the lab from napthalened is perfectly possible. Personally I'd just buy it, but
never mind. That part of the question has been answered.
Now let us ferret out the gremlins in the anthraquinone prep and the anthracene prep from that.
I will go get the Ber. citation and DOI for that paper and request from References.
[Edited on 11-11-2008 by Sauron]
Attachment: Pages from fundamental_processes_of_dye_chemistry.pdf (190kB)
This file has been downloaded 1036 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Org.Syn. Collective Volume 1 has two preps by the great Louis Fieser, the first of p-toluyl-o-benzoic acid, from phthalic anhydride and toluene with
AlCl3; the second treating that with hot fuming H2SO4 to obtain 2-methylanthroquinone.
These amply illustrate the identical reactions with benzene rather than toluene to yield 2-benzophenonecarboxylic acid (benzoyl-o-benzoic acid) and
from it, anthroquinone.
Now we only need to await the Clar paper from Ber. detailing the Zn reduction of anthraquinone to anthracene.
If no demons pop up, this tale will have been told.
I have combined the pair of Org.Syn.writeups into a single PDF for convenience and to keep vulture from flexing his talons at me for multiple
postings.
[Edited on 11-11-2008 by Sauron]
Attachment: CV1P0353.pdf (298kB)
This file has been downloaded 1643 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
We did not have a long wait for the Ber. paper thanks to vovan79.
Paper is attached. The zinkstaubschmelze = zinc dust melt. This is prepared from one equivalent of the anthraquinone, 1 equivalent NaCl, 5 equivalents
AnCl2, and 1 equivalent Zn dust in a melt at 210 C. Details on page 1647 at top. Anthracene is separated by sublimation and freed from a small
contaimnant of condensation product by recrystallization.
So that concludes this little adventure into polycyclic hydrocarbon land.
Napthalene -> phthalic anhydride
phthalic anhydride -> o-benzoylbenzoic acid
o-benzoyl benzoic acid -> anthraquinone
anthraquinone -> anthracene
All on your lab bench.
No really outre equipment, nor really exotic reagents.
I doubt it is going to get any easier than this. You can save yourself some bother by buying the phthalic anhydride. But the rest is rather
straightforward.
Have fun!
[Edited on 11-11-2008 by Sauron]
Attachment: Clar_Ber72_1645.pdf (360kB)
This file has been downloaded 950 times
Sic gorgeamus a los subjectatus nunc.
|
|
|
kazaa81
Hazard to Others
  
Posts: 368
Registered: 30-4-2004
Member Is Offline
Mood: ok
|
|
| Quote: | Originally posted by sparkgap
It fluoresces a nice blue, but back when I was working with anthracene, my real target was preparing triptycene (anthracene + a suitable benzyne
precursor like diazotized anthranilic acid). Fun.
sparky (~_~) |
yes but it seems to photodimerize under UV light, changing in physical properties; wiki says it can be reverted with UV below 300nm, though
sublimation could revert it as well if I'm right.
picture
@Sauron: thank you, I agree there couldn't be a simpler procedure.
[Edited on by kazaa81]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@kazaa81
You are welcome. Anytime.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Perhaps I should explain why I selected the phthalic acid route to anthracene for amatuer purposes, and rejected the two alternatives, i.e.,
napthoquinone and styrene dimerization respectively.
The napthoquinone method starts off much like the phthalic acid route with air oxidation of napthalene vaport over V2O5 on pumice support. The
differene is that throughput is increased to optimize for napthoquinone, although lots of phthalic acid is still produced and must be removed. That
seperation is objection No.1. Objection No.2 is the next step: Diels Alder with butadiene. Well, you could buy a cylinder of butadiene or you could
make your own using an apparatus essentially identical to a ketene lamp, q.v. in Org.Syn. The question is whether or not you would want to in a home
lab.
From that point themethod looks reasonably tame.
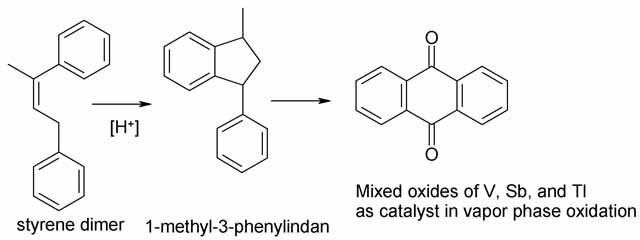
The styrene process takes a different long way round to get to anthroquinone, and has no apparent advantage over the others. The real objection from
my point of view is that the air oxidation to anthraquinone employs thallium oxide in addition to vanadium and antimony oxides, and I think thallium
oxide is simply too toxic to much around with in a home lab. Furthermore the product is impure and requires further workup before it can be reduced to
anthracene.
So proceeding from phthalic acid (DIY or commercial) is shorter. The Friedel-Crafts step in about quantitative. The cyclization requires 20% fuming
H2SO4 (20% SO3) but the lit says conc H2SO4 can be used with longer reaction times and lower yields.
[Edited on 13-11-2008 by Sauron]
Sic gorgeamus a los subjectatus nunc.
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I made some anthracene from benzene and phthalic anhydride. The phthalic anhydride can be obtained off of Ebay. The aluminum chloride is the problem.
This will have to be purchased from a chemical supplier. Alfa Aesar around $30 for a kilogram. The benzene will have to be made or purchased. I
recommend this lab to any one who is serious about amateur organic chemistry. Drain cleaner grade sulfuric acid can be used in the cyclization step.
The procedure is available for a gram scale synthesis in the williamson organic lab book. Sodium dithionate for the reduction step can be produced by
reducing sodium metabisulfite in an alkaline solution with zinc dust. Most of these chemicals can be obtained off of Ebay.
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I am planning on repeating part of this experiment soon. I have around 30 grams of o-benzoylbenzoic acid and some anthraquinone synthesized from the
benzoylbenzoic acid. Using the procedure in the Williamson textbook I plan to reduce the anthraquinone to anthrone with tin (II) chloride and then use
the zinc dust reduction to produce the anthracene. I synthesized the o-benzoylbenzoic acid from benzene and phthalic anhydride using an aluminum
chloride catalyst. I then used 92% sulfuric acid to close the ring to produce the anthraquinone. After adding water, the anthraquinone precipitates as
a light yellow-brown powder. I will be posting more information as I work on this experiment.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Nice work! Please do give us more deatails when possible!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by benzylchloride1
Using the procedure in the Williamson textbook I plan to reduce the anthraquinone to anthrone with tin (II) chloride and then use the zinc dust
reduction to produce the anthracene. |
I think, that one-step reducing of antraquinone by zinc/HCl (without tin (II) chloride) also must be possible.
|
|
|
Eclectic
National Hazard
   
Posts: 899
Registered: 14-11-2004
Member Is Offline
Mood: Obsessive
|
|
It certainly would not be selective for Anthracene, but does anyone have thoughts on just cooking toluene with AlCl3?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Just refluxing AlCl3 in toluene does nothing. Refluxing AlCl3 in toluene with prior saturation with HCl gas is supposed to lead toward Friedel-Crafts
transmethylation (methyl groups disproportionation) yielding xylenes and benzene, but I have yet to find a paper describing this. There are several
papers describing Friedel-Crafts transethylation and other transalkylations from ethylbenzene and other alkylbenzenes respectively (using HAlCl4,
HAlBr4 or HBF4 as acids), but the ethyl, isopropyl, not to even mention benzyl or t-butyl groups, transalkylate so very, very much easier
than the methyl. Therefore I have some doubts that toluene can even be transmethylated at all.
By the way, what does treating toluene with acids have to do with anthracene?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
May be, he mean the possibility of oxidative dimerization of toluene (like benzyl chloride) to 9,10-dihydro-anthracene, and then to anthracene.
|
|
|
benzylchloride1
Hazard to Others
  
Posts: 299
Registered: 16-3-2007
Member Is Offline
Mood: Pushing the envelope of synthetic chemistry in one's basement
|
|
I just ran the entire reaction from O-benzoylbenzoic acid to anthracene. The yield of anthraquinone from the O-benzoylbenzoic acid was around 45% due
to the acid being the monohydrate and the sulfuric acid was only 92%. Next time I plan on recrystallizing the acid from water and placing the dry
product in my rotovap and pulling a vacuum and heating the flask in a boiling water bath until the solid has a constant weight. The O-benzoylbenzoic
acid crystallizes as a monohydrate. The reduction from anthraquinone to anthrone went fairly well. The anthrone was obtained with a yield of about
82%. The reduction has to be followed exactly as stated in the Williamson textbook or else the anthraquinone will not be reduced completely by the tin
(II) chloride. I do not have sodium dithionate and so I did not use the dithionate reduction method. I think that the dithionate reduction would be
better because glacial acetic acid would not be required due to its expense. The reduction of the anthrone to anthracene worked well. The anthracene
was obtained as a yellowish crystalline solid that melted at 216 c, the literature value for anthracene. The yeild was around 50%, but I am still
working up the residual toluene that was used for cystallization. I just got my rotary evaporator to work and it has been a major help with this
experiment. I plan to use the anthracene to trap benzyne generated from anthranillic acid and isopropyl nitrite to produce the interesting cage
structured hydrocarbon, trypticene using the procedure in the Williamson textbook.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I came across a reference to Anthracene/Phenanthrene a few years ago whilst researching organic semiconductors.
At that time i had no idea of the Complexities involved, and followed a suggestion that it could be a component of 'partially oxidised organic
material', so i burnt some dry weeds in a metal bucket with some tinfoil (about 4" x 4") hanging from the bucket lid, which was put on after the weeds
caught fire.
After about 1 hour in the smouldering bucket, surprisingly i found a voltage of around 0.3V between the aluminium foil and two points on the tarry
coating in strong sunlight.
Unscientific i know, but it impressed me at the time.
|
|
|
Polverone
Now celebrating 21 years of madness
|
Thread Split
15-11-2014 at 12:55 |
|