| Pages:
1
2 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Strange manganese dioxide (?)
Having gotten a little tired of extracting MnO2 from spent batteries or from converting pottery grade MnCO3, I bought 500 g of "MnO2 - High grade
material" off eBay (to be fair, this seller sells various oxides and they've all got cut and pasted "High grade material" in their descriptions).
Disappointingly, this material isn't black, it isn't even all that dark, more a maroon reddish-brown.
A couple of tests: with an excess strong HCl it does generate chlorine and dissolves completely (to a greenish, presumably MnCl2 solution).
Adding Na2CO3 to this solution causes a whitish precipitate to form, presumably MnCO3. And adding an excess of hypochlorite causes the precipitate to
darken in colour but it seems to revert back to the colour of the original product, rather than go black. Adding peroxide to the oxidised precipitate
seems to cause the colour to darken a bit (but that could be subjective). I'll see what the precipitates looks like tomorrow.
The only surefire way to find out whether this is effectively MnO2 or another oxide (MnO, Mn2O3 or Mn3O4) would be to assay it (I don't know of any
quick and easy tests to determine the oxidation state of Mn in its oxides) for Mn content.
The colour would seem to point to Mn2O3 which I've read described as similar to Fe2O3.
Any suggestions?
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
The manganese-dioxides have several modifications; some are eg. grown on others (as substrate) when battery-making-MnO2 is produced, it's a science of
it's own, numerous patents out there.
Depending on the source it can be any sort of mixture of any modifications of MnO2, and the label "MnO2" doesn't even tell much about the chemical
properties; for chlorine-generating everything might be fine, but performance in an electrochemical cell is a different matter.
The most common way, in a real lab, is to x-ray the stuff (powder diffraction); each structure has it's characteristics.
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
Blog, I believe what you have is hydrated MnO2.H2O approx. Essentially it may be pure enough. For your suspected use (thermite) heat can rid you of
the H2O, but not too much. Look up decomposition temps for MnO2 - around 500C IIRC. Chemically there may be nothing wrong with it - the hydrated form
is a dark brown, the ore black. Heating the hydrated carefully should turn it black...
Der Alte
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@Chief and DerAlte:
Yes, the exact structure of MnO2 depends on conditions of production, I'm aware of that but I've never seen a product labelled MnO2 that's so light in
colour that it can't even remotely be described as black. My own preparations (from oxidising freshly precipitated Mn(OH)2.n H2O or MnCO3 with bleach)
tend to vary somewhat too and usually after calcining (at 400 - 500 C) take a very dark, 'pure chocolate' brown, rather than the familiar black
(described by many about MnO2).
The dioxides precipitated in test tubes yesterday afternoon have darkened a lot upon standing for about 16 h (in one, the most alkaline test, the
supernatant liquid contains a small amount of MnO<sub>4</sub><sup>-</sup> as evidenced by the typical colour - I've
inadvertently made small amounts of permanganate quite a few times - lol). There is no question that the starting material is a compound of mainly of
manganese and oxygen.
DerAlte, interesting suggestion on MnO2.H2O. Acc. Wiki the decomposition of the dioxide to Mn<sub>2</sub>O<sub>3</sub> in air
occurs below 800 C and my first idea was that someone had perhaps calcinated their crude (wet) MnO2 a <i>liiiittle too fervently</i>,
driving it to Mn (III). But perhaps they've calcinated it too gently?
But if you're right about MnO<sub>2</sub>.H<sub>2</sub>O, then corresponding weight loss should show up at 400 - 500 C. I'll
try that tonight but somehow my hopes aren't high. Still, must keep an open mind...
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
If your solution gives a whitish precipitate with Na2CO3 then your source of Mn is quite pure and is a good grade. I also have "MnO2", a crystalline
black powder, but its solutions when reduced with e.g. Na2SO3 gives brown precipitates with Na2CO3, indicating that it contains a lot of iron. So, I
have the feeling that your purchase was a good one. If you want to use it for thermite, then you might need to heat the stuff to get rid of water, but
for aqueous chemistry this materials probably is very good.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
2 hours @ 400 - 500 C and the weight change is 0.6 w%, not worth talking about (considering also measuring error of at least 0.05 w%). No change in
appearance either, while hot it looked a little darker (subjectively) but cooled down it's "like new".
It's puzzling. I'll have assay for Mn content.
Woelen, a pottery grade MnO2 I assayed once contained more than 20 % Fe (expressed as Fe2O3) and a few percent acid insolubles, presumably siliceous
matter. The precipitates clearly showed that, as per your own experience...
|
|
|
DerAlte
National Hazard
   
Posts: 779
Registered: 14-5-2007
Location: Erehwon
Member Is Offline
Mood: Disgusted
|
|
Puzzling, Blog. You are obviously familiar with the darkish brown precipitated MnO2.xH2O (it even looks black in incadescent light sometimes) and you
say it is a much lighter brown. Sounds like Mn2O3, which I have made by subjecting MnO2 (battery impure, contains carbon + other shit) and subjecting
it to a high red heat. Yet CRC says that Mn2O3 is black; Mn3O4 they say is brown, but I don't think I heated that high.
The only way to determine may be to react with HCl (all the oxides do, in varying amounts) to produce MnCl2 (and Cl2, of course, bar Mn0), precipitate
out with NaClO to give hydrated Mn02.xH2O weighing initial and final products. Sounds tedious!
I agree with Woelen that pottery grade contains large amounts of Fe. The carbonate produced the way he says may be slightly pink. It it is
precipitated brownish or yellow, Fe is certainly the culprit. The Mn in alkaline cells is pretty pure, IMHO. Not so in the older C/Zn cells.
Der Alte
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
DerAlte:
Yep, I'm flabbered. On the face of it (colour) this stuff could easily be confounded with red iron oxide.
On the colours of these oxides there seems to be some dispute...
Next stop: assay for Mn (as carbonate). Tedious is the word alright...
I'm not really <i>too</i> bothered which oxide it actually is but need to know for stoichio reasons.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Every sample of MnO2 I've seen (most of them through decomposition of dissolved KMnO4) was dark brown. MnO2 from batteries is black because it has
been mixed with graphite.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Vulture:
That's how I see it too: my homemade MnO2, either from battery crud (separating the graphite chemically) or from pottery grade MnCO3 has always been a
very dark brown, bordering on black.
But many maintain it's supposed to be completely black. Me? I don't know anymore and this latest purchased batch is thoroughly confusing, this colour
just doesn't fit.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I've finally gotten round to analysing the strange reddish 'MnO2' but haven't got the complete results yet.
But I carried out two more tests on the suspect MnO2 and some homemade MnO2.
An old chembook tells me that Mn<sub>2</sub>O<sub>3</sub> dissolves in dilute H2SO4 according to:
Mn2O3 + H2SO4 ---> MnSO4 + MnO2 + H2O
so I decided to try that with both the suspect MnO2 and some homemade MnO2.
I put a small pinch (less than a gram) of both in separate test tubes and added about 3 cm of 50 w% H2SO4 to each. Nothing happened, no dissolution,
no colour change, not even on standing overnight. But when I boiled up both tubes today, there was an immediate change. I boiled for a few minutes,
then let stand, cool and settle.
The results in the case of the suspect MnO2 were quite revealing. Imagine, if you will, the settled test tube to look like this: at the very bottom
about 1/2 cm of a green (darkish, but green nonetheless) precipitate, on top of that about the same amount of a black/grayish precipitate. On top of
the precipitates, supernatant liquid with the unmistakable purple of permanganate (quite deeply coloured too)...
The only green manganese compounds I know of are MnO (Mn (II) oxide) and manganate compounds (MnO<sub>4</sub><sup>2-</sup>, Mn
(VI)). As the green substance is clearly insoluble, MnO seems to be the most plausible species (even though it would unusual in strong acidic
conditions). Also, MnO<sub>4</sub><sup>2-</sup> is only stable in strongly alkaline conditions...
The test tube with the homemade MnO2 looks different: there's no black/grayish layer.
If I assume the suspect MnO2 to be rather Mn<sub>2</sub>O</sub>3</sub> than MnO2 I'd have to conclude that some strange
disproportionations had taken place in these very strongly acidic conditions:
5 Mn (III) ---> 4 Mn (II) + Mn (VII)
And to explain the black layer:
3 Mn (III) + Mn (VII) ---> 4 Mn (IV)
and/or:
3 Mn (II) + 2 Mn (VII) ---> 5 Mn (IV)
and maybe:
2 Mn (III) ---> Mn (II) + Mn (IV)
I should be able to put up some photos tomorrow.
[Edited on 26-10-2008 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Here's some pix:
Left: homemade MnO2, right: the bought 'high grade MnO2'

Test tube #1 with the bought 'high grade MnO2', after boiling with 50 % H2SO4

Test tube #2 with the homemade MnO2, after boiling with 50 % H2SO4

Poor colour representation in all cases due to the flash but the green and permanganate purple can clearly be discerned.
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Is your "high-grade MnO2" really that light? It looks almost orange!
The purple color can be understood. It is the color of Mn(3+) in very strongly acidic solution with a coordinating ion. This even is a test for
manganese.
I have a solid with manganese(III) in it, it is a phosphato-complex, NH4 [MnP2O7], ammonium pyrophosphatomanganate(III):

If this is dissolved in strong acid, a deep purple solution is obtained. I can imagine that your purple color is due to some sulphato complex given
the very high concentration of sulphuric acid.
All these manganese-things also are puzzling me, it is not what I expect. The pale green color also is not what I expect, nor the grey stuff.
If you have KMnO4, then you could try a similar experiment. First make MnO2 from the KMnO4, rinse, and dry. Then add that to the 50% H2SO4 and repeat
the experiment. In this way you are 100% sure that no other metals are in play.
EDIT by woelen: Changed link, so that it works again.
[Edited on 12-6-12 by woelen]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@ Woelen:
The colour of the brown-reddish oxide to the naked eye is darker than the photo shows.
I was going to test the supernatant solution for permanganate but haven't found time yet. So you believe it's a Mn<sup>3+</sup> complex,
<i>huh?</i> Very interesting indeed. Seems strange to me that the homemade MnO2 shows it too but it's far from impossible of course.
Filtrating off and gently alkalising the purple solution may precipitate Mn<sub>2</sub>O<sub>3</sub>.n
H<sub>2</sub>O, what say you? Or will it disproportionate?
Is your pyrophosphatomanganate(III) in solution capable of oxidising chloride to chlorine, as permanganate is? That would be one way of distinguishing
the two...
[Edited on 27-10-2008 by blogfast25]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Yes, the manganese(III) complex I have oxidizes conc. HCl to Cl2, so that unfortunately is not a distinction. A good distinction however is to add a
few drops of the purple solution to a strongly alkaline solution. Permanganate ion is not stable at VERY high pH (pH > 14), it looses oxygen and
the solution turns dark green, with formation of manganate. This reaction is quite sensitive, as the color of manganate is very intense, just as the
color of permanganate. A manganese(III) compound definitely will not show such behavior, it will indeed form brown Mn2O3.nH2O as you suggest.
Keep in mind that ANY sample of MnO2 will be oxygen deficient. Even the best samples in the world either are of the form MnOx, with x < 2
(somewhere around 1.95), or of the form MyMnO2, where My is a metal in low proportion, e.g. potassium with index y=0.05 or so. This effectively means
that the sample is MnOx, contaminated with some K2O, such that the oxygen comes exacly to 2, but some potassium ions are in the sample. All of this
means that there ALWAYS is some Mn in the +3 oxidation state. Really pure MnO2 apparently does not exist.
More information about this is available in the book "Chemistry of the Elements" by Earnshaw and Greenwood. This is interesting stuff and it may be
interesting for you to know that all real-world samples of MnO2 always are somewhat oxygen deficient.
[Edited on 27-10-08 by woelen]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Unfortunately my home assay of the bought 'MnO2' (based on dissolution in HCl and re-oxidation and re-precipitation as MnO2) wasn't conclusive, some
precipitate must have got lost along the way. Really only a titrometric method could save me here but I don't recall any (for
Mn<sup>2+</sup> . .
I'm aware of the fact the MnO2 is always somewhat oxygen deficient.
Later on today I'll alkalise the supernatant solutions and see if Mn2O3 (Mn III) precipitates or whether manganate (Mn VI) forms.
The difference in behaviour between the two oxides in boiling 50 % sulphuric acid would seem to suggest they are distinctly different species...
[Edited on 28-10-2008 by blogfast25]
Well, I dropped about 1 ml of the supernatant fluids in a few ml of 5 M NaOH and both behaved similarly. Firstly a reddish-brown colour change is seen
but without precipitation. Then after cooling back to RT and on adding a few more ml of 5 M NaOH, slowly a whitish gelatinous precipitate forms. At
first this precipitate is almost transparent (like freshly precipitated waterglass). No green colour was observed at any time.
I'm to assume the Mn2O3 in these conditions is either very lightly coloured or disproportionated to Mn(OH)2 and x (?).
But it shows at least no permanganate was present...
I'll repeat these tests, this time using dilute H2SO4 (say 1 M) instead of 50 %...
[Edited on 28-10-2008 by blogfast25]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ blogfast
Are you sure what you have is not Mn3O4 (naturally - hausmanite). Manganosic oxide is a brownish-red colour, (often called red oxide of manganese). It
is formed when any other manganese oxide is heated in air.
According to Mellors;
When heated with dilute sulphuric acid a soluble manganous salt and an insoluble hydrated manganese dioxide are formed.
When reacted with cold, concentrated sulphuric acid a mixture of manganous and manganic sulphates form.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@Xenoid:
I haven't been able to exclude Hausmannite, no. From what I gather it's usually darker than my stuff but bar a complete chemical analysis (or X-ray
diffraction) no oxides can be safely excluded with the exception perhaps of MnO (monoxide).
And Mn3O4's reactions don't appear very much discernible from Mn2O3's...
[Edited on 28-10-2008 by blogfast25]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Yes, what you may have is a mixture of oxides, possibly originally crude MnO2 (pyrolusite) which has been "roasted" to "purify" it, and in the process
generating Mn2O3 and Mn3O4. Often pottery grade material is little better than crude ore.
I recall inadvertently picking up some "iron chromate" with little though, whilst at a pottery store. The material turned out to be powdered chromite
ore, (FeCr2O4).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Actually it was sold to me as 'high grade MnO2'. It's certainly quite pure: 100 % HCl soluble and no Fe contamination either but pure in what??
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
It is pure in the sense that it only contains Mn and O (and possibly some water), albeit in a somewhat uncertain proportion. This purity is a very
good thing. Many MnO2-species contain quite some Fe, and also SiO2, leaving behind insoluble matter.
The fact that no dark green material is formed in a highly concentrated solution of a hydroxide makes me more sure that the purple color indeed is due
to the presence of Mn(3+).
This probably will be one of my little weekend projects next weekend. It is too interesting to just let this go...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@Woelen:
Great!
Here are the strange precipitates obtained after dropping some of the purple supernatant fluids into 5 M NaOH:

(Left #1, right #2) Not really what I expected either: doesn't really look like 'Mn2O3. x H2O', does it? The precipitates have already darkened
slightly since yesterday. Most of yesterday you could practically read the newspaper through them!
And I found this titrimetric determination of manganese in steel:
AS/NZS 1050.14:1994:
3 PRINCIPLE The sample [steel] is dissolved in dilute sulfuric acid, any other acid additions necessary for complete dissolution being removed by
fuming after oxidation, and interfering elements removed by precipitation using a zinc oxide suspension. The resultant solution is strongly acidified
to prevent interference from cobalt and the manganese is oxidized to the permanganate ion by ammonium persulfate. The permanganate ion is titrated
with ammonium iron(II) sulfate and potassium dichromate, using preoxidized sodium diphenylamine sulfonate as indicator.
Unfortunately half of the paper is available for purchase only...
********
I boiled both precipitates in a steambath. Also in the photo: 3 g of the unknown oxide being boiled in a conical flask with 100 ml of 1 M
H<sub>2</sub>SO<sub>4</sub> (ooops - must clean that cooker again!):

Left and right: #1 and #2 after a few minutes of boiling. They've lost a lot of volume (water) but remain light in colour, not very indicative of
Mn2O3. x H2O.

The remainder of #2 (see post above), after simply adding DIW to the rim of the tube but without mixing. The green Mn compound can be seen clearly. To
the naked eye it's much lighter...

The now diluted supernatant liquid (above) of #2 was then carefully siphoned off into a small conical flask. This presumably is the relatively pure Mn
(+III) compound. It didn't suffer hydrolysis after dilution (about (2:1).

The unknown oxide, when boiled with 1 M H<sub>2</sub>SO<sub>4</sub> turned black almost immediately. I simmered for about 15
min, then cooled, settled and filtered. It filters difficultly (runs through the filter, at least much of it). The filter was treated with hot 32 w%
HCl: the (truly) black precipitate dissolves with evolution of chlorine. Presumably it is MnO<sub>2</sub>.
The filtrate is now decanting but I can already see the supernatant liquid to be clear and colourless: dilute MnSO<sub>4</sub>?
Any ideas on what tests to perform on the green compound?
*********
The clear, now decanted filtrate precipitated a creamy, white precipitate when saturated Na<sub>2</sub>CO<sub>3</sub> was
added (after cessation of bubbles). Adding bleach to the precipitate and it turns brown/black. Presumably the clear filtrate contained
MnSO<sub>4</sub> and the precipitate was MnCO<sub>3</sub>, then oxidised to MnO<sub>2</sub> with hypochlorite.
So it's safe to say the unknown oxide reacts with dilute sulfuric acid according:
y Mn oxide + H<sub>2</sub>SO<sub>4</sub> ---> x MnSO<sub>4</sub> + z MnO<sub>2</sub> (+ water?)
That would be consistent with Mn<sub>2</sub>O<sub>3</sub> but apparently also with
Mn<sub>3</sub>O<sub>4</sub>...
And another (but similar) method for titrimetric determination of managnese (free article)
It would be interesting to use this method to determine the proportions of Mn (II), Mn (IV) and Mn (III) obtained in various conditions of
dissolution.
The method essentially calls for oxidising the Mn to permanganate with amm. persulfate, then adding a standardised excess of
Fe<sup>2+</sup> and back titrating the excess with standardised KMnO<sub>4</sub>:
Mn (II) + persulfate ---> Mn (VII)
Mn (VII) + x Fe (II) ---> Mn (II) + 5 Fe (III) + (x-5) Fe (II)
(x-5)/5 Mn (VII) + (x-5)Fe (II) ---> (x-5)/5 Mn (II) + (x-5) Fe (III)
[Edited on 29-10-2008 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Some more experiments...
1) On the white precipitate obtained from the purple solution + alkali:
Firstly, the precipitates (3rd pic, previous post) that had lost so much volume had reconstituted themselves overnight:

The swollen precipitates dissolve in cold, strong HCl but not eagerly and a clear solution was obtained. I didn't notice any chlorine... Also
remarkable is the fact that when I neutralise this obtained clear solution and then add sat. sodium carbonate solution, no precipitate is formed. Only
by adding more NaOH (5 M) does a gelatinous precipitate eventually show up. This is a clear indication that the solution does not contain much, if
any, Mn<sup>2+</sup>.
Another portion of the precipitate was subjected to bleach (hypochlorite, at pH 13 - 14) and a steam bath: hardly any oxidation at all took place:
there is no colour change to black, so typical when bleach is added to freshly precipitated Mn(OH)<sub>2</sub>.
2) On the green precipitate:
After having siphoned off the purple liquid from both test tubes, I added 32 w% HCl to the green precipitates: both reacted very quickly, turning
black, with generation of chlorine and a lot of heat, the test tubes became too hot to hold:

The black stuff (combined from both tubes) with added strong HCl and some heat quickly clears to MnCl<sub>2</sub> and chlorine (lots of
it):

3) On the purple solution, suspected to contain Mn (+III):
Adding oxidisers:

Left: the purple solution as such.
Middle: after adding bleach. It slightly darkens. No precipitate. We're at pH ≈ 0 though...
Right: after adding weak H<sub>2</sub>O<sub>2</sub>. Goes clear.
Adding reducers:

Left: after adding strong HCl. It darkens considerably, no chorine though. MnCl<sub>3</sub>? Which is allegedly dark coloured but should
lose colour through decomposition to MnCl<sub>2</sub> on heating (like MnCl<sub>4</sub> does).
Edit: after heating the solution does clear up and chlorine is formed during that. That would strongly point to
MnCl<sub>3</sub>... Even a saturated NaCl solution (about 6 M, 32 w% HCl is about 10 M) added to the purple solution caused it to darken
slightly, which disappeared on heating and the faint smell of chlorine could be observed. This would point to MnCl<sub>3</sub> being
analogous to MnCl<sub>4</sub> in being more covalent than heteropolar and unstable at higher temps.
Middle: after adding bisulfite. Clears up completely. Presumably reduces Mn(III) to Mn(II).
Right: after adding strong Fe<sup>2+</sup>. Clears up. Presumably reduces Mn(III) to Mn(II). The residual green is due to excess
Fe<sup>2+</sup>, IMHO.
Some more to follow.
I read that Mn<sub>2</sub>(SO<sub>4</sub> <sub>3</sub> forms alums with Rb and Cs, albeit not very stable ones. I wonder if it would be possible to pull a potassium alum from the
purple solution... It'd be nice to have a solid Mn(III) bearing compound... <sub>3</sub> forms alums with Rb and Cs, albeit not very stable ones. I wonder if it would be possible to pull a potassium alum from the
purple solution... It'd be nice to have a solid Mn(III) bearing compound...
[Edited on 30-10-2008 by blogfast25]
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Wow, you have done a lot of experimenting. Let's try to organize things a little...
- concentrated acid vs. dilute acid
- presence of chloride ion vs. presence of sulfate only
- use of reductor
- use of oxidizer
- ...
By organizing all things, a pattern may appear.
Some remarks which I can give already: the strong heat you observe by mixing the purple liquid with conc. HCl most likely is due to heat of hydration
of conc. H2SO4. The purple liquid contains a lot of H2SO4 and hardly any water.
------------------------------------------------------------
I also have done some experimenting by now, this weekend I'll have more time.
Experiment 1: Add black MnO2 (laboratory grade material, not from some eBay seller or some pottery shop, but a real lab chem) to a 1 : 1 mix of conc.
H2SO4 (also reagent grade) and distilled water. Heat this stuff.
The result of this experiment is no reaction at all. The black solid does not dissolve. I kept on boiling until white smoke was produced from the
sulphuric acid. The solid does not dissolve.
Experiment 2: Use the same setup, but now add a small amount of solid Na2SO3, such that some of the MnO2 could be reduced. When this is done, then
some SO2 is produced (pungent smell!!), but the liquid also turns bright purple. When the liquid is allowed to stand for a while, a nice bright purple
liquid above a black solid appears. So, the purple material really is due to some lower manganese compound (most likely +3 oxidation state).
When water is added to the purple liquid, then it becomes turbid. After one day of standing, a black precipitate is formed, with a nice brown/red
liquid above it. This color is due to Mn(3+) in aqueous solution at high concentration of water. This color is familiar to me.
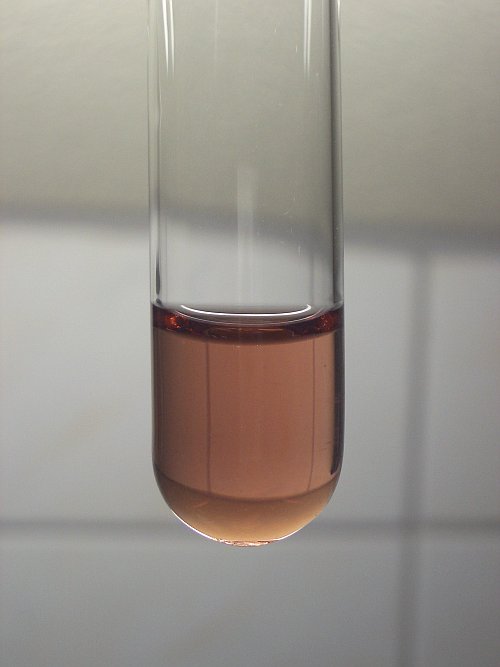
---------------------------------------
In another experiment I took MnSO4 and dissolved this in 3% H2O2 and added this to excess 5% NH3. A dark brown flocculent precipitate is formed. I'll
let this settle to the bottom and clean this, such that next weekend I have some material to play with. This brown flocculent precipitate most likely
is hydrous MnOx, with x < 2.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by woelen
Some remarks which I can give already: the strong heat you observe by mixing the purple liquid with conc. HCl most likely is due to heat of hydration
of conc. H2SO4. The purple liquid contains a lot of H2SO4 and hardly any water.
|
It's possible but the amount of 50:50 (NOT conc. H2SO4) accompanying the green precipitate was very small compared to the amount of strong HCl added:
remember that I had siphoned off the supernatant purple liquid as best as I could. Diluting 50:50 H2SO4 does give off heat of course but in my set-up
that heat should have been adequately absorbed by the excess HCl, IMHO.
I'll repeat that experiment by washing off the remaining 50:50 H2SO4 first, on a bigger scale...
One other thing I must mention is that my H2SO4 contains small amounts of Fe (as Fe<sup>3+</sup>, I believe).
Strange that you do not observe what I see with my homemade MnO2, because despite it being a home production, it's quite pure.
Look forward to your next results...
|
|
|
| Pages:
1
2 |