stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
Methylamine/Ammonia, how to tell them apart?
i recently bought a bottle of commercial 40% Methylamine solution in Methanol. Now after opening, it struck me that what i may have here is in fact a
solution of Ammonia in Methanol, because the smell is so much different than the rotten pussy stench i was used to from the aqueous solutions of
methylamine.
is there a quick qualitative test to discern between ammonia and methylamine that could be used to determine if the supplier delivered a mislabeled
bottle?
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
boiling point? methylamine b.p -6*C ammonia b.p -33*C ... should be easy to tell if you pass the vapours through a cold trap whether it is one or the
other if you know the temperature of the cold trap would only allow methylamine to condense.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Mayeb you could try acidifying an small aliquot, evaporate the methanol, and test the solubility in IPA?
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Flame test; methylamine is much easier to ignite than ammonia (it has lower and wider flammability limits). If you want to get tricky pass combustion
product through milk of lime to test for CO2 which ammonia of course will not produce.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
| Quote: | Originally posted by stoichiometric_steve
i recently bought a bottle of commercial 40% Methylamine solution in Methanol. Now after opening, it struck me that what i may have here is in fact a
solution of Ammonia in Methanol, because the smell is so much different than the rotten pussy stench i was used to from the aqueous solutions of
methylamine.
is there a quick qualitative test to discern between ammonia and methylamine that could be used to determine if the supplier delivered a mislabeled
bottle? |
Just run it past the g/f!
Does this smell like rotten pussy?
Reckon on a substantial loss of china and glass plus cases being packed and a decampment to her mother's for a few weeks 
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
CH3-NH2 would have a C-N bond, with the C and N bonded only to Hs, unlike CH3OH and NH3. So, if you have a scanning infrared spectrometer, a C-N
absorption band would show up if any CH3NH2 is present, and the wavelength would be at the short end of the IR absorption of C-N bonds.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
How about sacrificing a few cc to make the hydrochloride, then evaporate to dryness. From The British Pharmaceutical Codex
| Quote: | METHYLAMINE HYDROCHLORIDUM.
METHYLAMINE HYDROCHLORIDE.
CH6NCl = 67-508.
Methylamine hydrochloride, CH3NH2,HCl, is prepared by neutralising an aqueous solution of methylamine with hydrochloric acid, and evaporating to
dryness. The residue dissolves readily in boiling alcohol, which, on cooling, deposits the salt in crystals. These crystals at the
moment of their formation appear iridescent by reflected light.
It occurs in fine, large laminae, colourless, and deliquescent. Very soluble in water and in alcohol ; insoluble in chloroform. Its solubility
in alcohol distinguishes it from ammonium chloride. Melting-point, 222°. Heated in an open vessel to a high temperature, it volatilises in
dense vapours, which condense to a white powder on cold surfaces. It forms with many metallic chlorides well- crystallised double salts.
|
From the OrgSynth entry, 800-900 g of dry MeHH3Cl should dissolve in 2 liters of boiling absolute ethanol. It will be a somewhat less soluble in
isopropanol, but not more than half as much, and ammonium chloride is nearly total insoluble in i-PrOH.
Also https://sciencemadness.org/talk/viewthread.php?goto=lastpost...
[Edited on 9-10-2008 by not_important]
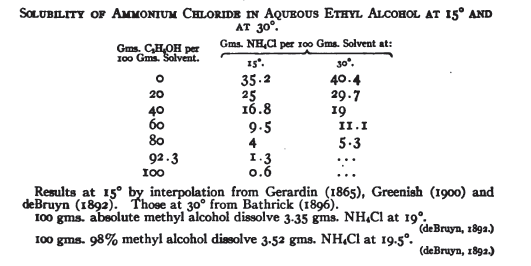
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
pure meam smells a lot like ammonia but I like the suggestion to employ a few drops of HCl on a small sample. The crystals are shinier and more
platelike than NH4Cl. Very hygroscopic .. in a humid atmosphere they will disappear in a puddle. And then you can always do phys. props. of the
sample.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
i don't believe a 40% solution in methanol is possible either for methyl amine or ammonia. Who did you buy it off, www.iamdodgeychemsupplier.com?
40% MeAm in H20, the highest possible at STP has a bp of 48C.
[Edited on 9-10-2008 by Panache]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Panache
i don't believe a 40% solution in methanol is possible either for methyl amine or ammonia. Who did you buy it off, www.iamdodgeychemsupplier.com? |
So you consider Abcr, one of the biggest chemical suppliers in Europe, a bit dodgy to offer 40% methylamine solution in methanol for sale:
http://shop.abcr.de/product_information.aspx?product_id=3504...
(There are a few other small suppliers that also sell it. It is however true that Sigma does not, but they do sell 33% solution in ethanol.)
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
The stuff i have is from TCI Tokyo Kasei (cheapest supplier for this item that i know of, 26 EUR per 500ml vs. 79 per 100ml from ABCR). I have now put
some HCl in IPA on a few drops of the methanolic solution and it turns out the stuff is indeed very deliquescent, as opposed to a sample of NH4Cl.
It is peculiar that the smell of methylamine obviously depends very much on solvate molecules. Alternatively, the 40% aq. solution i have (from Acros)
may be contaminated with traces of dimethylamine that cause the pussy smell. It really makes me sick to smell the aqueous stuff, while the methanolic
solution doesn't bother me nearly as much...
[Edited on 10-10-2008 by stoichiometric_steve]
[Edited on 10-10-2008 by stoichiometric_steve]
|
|
|
Ephoton
Hazard to Others
  
Posts: 463
Registered: 21-7-2005
Member Is Offline
Mood: trying to figure out why I need a dark room retreat when I live in a forest of wattle.
|
|
heat some of the solution and drip hcl into it. if you get a white fluffy percipitate you have ammonia piss easy
the smell is normal for nitromethane being reduced in alcohol the fish smell telling the chemist of the existence of excess water.
[Edited on 10-10-2008 by Ephoton]
e3500 console login: root
bash-2.05#
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
yeah i get it now, close the thread
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
| Quote: | Originally posted by Nicodem
| Quote: | Originally posted by Panache
i don't believe a 40% solution in methanol is possible either for methyl amine or ammonia. Who did you buy it off, www.iamdodgeychemsupplier.com? |
So you consider Abcr, one of the biggest chemical suppliers in Europe, a bit dodgy to offer 40% methylamine solution in methanol for sale:
http://shop.abcr.de/product_information.aspx?product_id=3504...
(There are a few other small suppliers that also sell it. It is however true that Sigma does not, but they do sell 33% solution in ethanol.)
|
hmmm, perhaps i should have checked other sources beyond the Sigma catalogue in front of me. Apologies all, the dodgey comment pertained to the fact
that steve seemed to think his company had such poor systems they could mislabel one item as another but written after my questioning whether such a
product was even possible implied that it was their marketing that was dodgy.
Basically a useless post on all front.
Apologies.
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Mislabeled products are not that uncommon, even with leading products. Especially with products that look alike. The problem doesn't come from the
production obviously, but the labelling of the bottles. Out of all the mislabled items, some of them get through the checkups and are then sent away.
Sometimes you get lucky, other times you don't!
\"You can battle with a demon, you can embrace a demon; what the hell can you do with a fucking spiritual computer?\"
-Alice Parr
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
yeah but GUYS, it's already cleared up, thanks!
|
|
|