Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
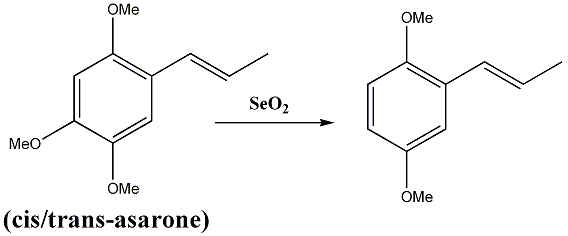
Deoxygenation of (cis/trans)-asarone using selenium dioxide.
A very interesting paper [1] came to my attention regarding the deoxygenation, in para position exclusively, of a mixture of asarone isomers. The
procedure is remarkably mild and involves the use of selenium dioxide. Unfortunately, the authors are not very explicit about the theoritical aspect
of this result.
I have researched a lot on the possible mechanism, as well as the possibility to extend this procedure to other similiar systems. I was wondering if
anyone could provide me with any (experimental or theoritical) information regarding such selectivity.
Many Thanks in advance.
[1] Rao et.al., J. Chem. Soc. 1338 (1937)
[Edited on 23-9-2008 by Quantum_Dom]
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Careful with SeO2!!!
MSDS:
This is a particularly dangerous chemical. Highly toxic. May be fatal if inhaled, swallowed or absorbed through the skin. Possible mutagen. Vesicant.
Danger of cumulative effects. Note low LD50s below. May damage CNS. If trapped under finger or toe nails very severe pain results. Note that the LD50s
given below are much lower for skin contact than those for ingestion; a few tenths of a gram in contact with the skin may be fatal. Suitable
protection is essential! Check integrity of gloves before use. Very destructive of mucous membranes. May harm liver, spleen, G.I. system.
http://msds.chem.ox.ac.uk/SE/selenium_dioxide.html
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Thanks for this Jor, I must say that I already know a lot about SeO2 toxicity. Not a problem.
[Edited on 22-9-2008 by Quantum_Dom]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I doubt this is useful on a preparative scale...
Here is the original paper...
Attachment: rao_beta_asarone.pdf (263kB)
This file has been downloaded 949 times
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Thanks for the paper. Would you please elaborate on why do you think this would not be useful ?
Although the authors use 20 grams of asarone, I do agree that they do not report the yield of 2,5-dimethoxypropylbenzene obtained.
Yet, I do not see any information that would lead someone to believe that it is not a viable and convenient procedure.
[Edited on 22-9-2008 by Quantum_Dom]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
This seems like one of those papers where they are just trying to characterize a compound; There is a good chance that if you follow the procedure you
will get 2,5-dimethoxypropenylbenzene but I doubt you will get very much. It is just a hunch, because for the first reaction they gave a yield (which
was pretty good) but for the second they did not. Also selenium(IV) is an oxidizing agent and in this scheme it is reducing B-asarone. Additionally
the stoichiometry of this reaction makes no sense.
Of course, what are my .02 cents worth? If you trust the method give it a try and be sure to post your results  . .
Of note I have read old papers where they definitely screwed up the characterization of a product. For example I was reading a paper trying to
determine the structure of tryptophan and the authors said, essentially, we are sure this is the structure of tryptophan, but they were dead wrong
(they had the COOH in the 2 position of the indole nucleus).
[Edited on 9-22-2008 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
LOL, yeah I agree theres a lot of trash out there. You are right to be careful.
Its just that March's advanced organic chemistry discuss this phenomena very lighltly with no theory whatsoever and gives a much complicated
reference that uses exotic reagents [1].
[1] March's advanced organic chemistry, 5th edition, p734
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
What is the name of the chapter that describes it; I must have a different version
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Aromatic electrophilic substitution
The subsection is entitled: Deoxygenation.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Eh, its not in my version; If you don't mind what did march have to say and what was the reference?
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Np, thanks for considering this thread. Youre interest is much appreciated  . .
| Quote: |
In a few cases, it is possible to remove an oxygen substi. from the aromatic ring [1]. ArOR ======> ArH where Ar:aromatic ring
|
[1] Sasaki, K Chem. Lett, 1997, 617
[Edited on 22-9-2008 by Quantum_Dom]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Well, A quick skim of the refrence shows much different conditions. Namely the substrate is a mesylate and the conditions of the reaction are
reducing.
Reductive cleavage of methyl phenyl ethers is known however I have never seen it done selectively.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Yeah, I figured that reference was kind of obscure and a bit misleading.
Would it be possible that SeO2 is only acting as a Lewis acid here and not as an oxidizer ?
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
So even if SeO2 was only acting as lewis acid, it doesn't change the fact that in the overall process the asarone is getting reduced. I suppose
ethanol could be the reducing agent coupled with SeO2 catalysis, but this is just a wild guess.
Concerning the reaction: the authors of the title paper summed it up best.
| Quote: | If the heating is prolonged, a complex mixture of products is formed from which 2 : 5-dimethoxypropenylbenzene
has been isolated. |
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Complex mixtures: I dont like the sound of that ! 
Many thanks for your help smuv! I appreciate it a lot .
[Edited on 22-9-2008 by Quantum_Dom]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Deoxygenation with SeO2, which presumably would end up as SeO3 or H2SeO4? But SeO3 and selenates are reasonably strong oxidants, which means that the
reaction, if it is possible, would have to be endothermic, and the Se(VI) removed from the reaction mixture as soon as it is formed. The same reaction
using SO2 instead would be much more likely to be successful.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I'm thinking...the Se(VI) would oxidize ethanol (the solvent) to regenerate the SeO2. Although I dont see how this could mechanistically occur
(oxidation of SeO2) to begin with.
EDIT: I realize I wrote Se(IV) instead of Se(VI) sorry for any confusion.
[Edited on 9-22-2008 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
What about the presence of oxygen within the reactionnal flask ? Could presence of O2 actually oxidize Se(IV) to Se (VI) ?
[Edited on 22-9-2008 by Quantum_Dom]
[Edited on 22-9-2008 by Quantum_Dom]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The original reference describes a weird method of cis/trans isomerisation of beta-asarone to asarone using SeO2. Prolonging the reaction gave them a
mixture of crap from which they claim to have isolated 2-5-dimethoxypropenylbenzene characterized on elemental analysis, bp and picrate complex mp.
and further derivatization (no spectroscopic analyses, but that is normal for the year 1937). The bp does not come close enough to the one reported by
other sources (reported as 145-150°C at 8 Torr; instead of 126°C at 13 Torr for 2,5-dimethoxypropenylbenzene). They do not give a mp literature
reference for the picrate complex and I could find no such complex described anywhere. The elemental analysis is done for a picrate but 10.1% N means
little due to the high MW of the picric acid compared to the nitrogenless substrate. The elemental analysis for the nitrosite fits well but it could
just as well fit for other stuff. I would not rely much on elemental analysis data, especially if reported only for N. The mp of the nitrosite they
give (118°C) does not match the literature value other authors reported (130°C, dec.; JOC, 18, 1253-1262). The 4-propyl-2,5-dimethoxynitrobenzene
derivative is to my queries an unknown compound and they give no mp literature reference so it is beyond my imagination on how they managed to claim
identity.
But even if they really got some 2,5-dimethoxypropenylbenzene, they do not say how much and beyond the immense mechanistic curiosity that this would
represent, such a reaction would obviously be of no preparative use as is now (especially considering that 2-5-dimethoxypropenylbenzene is much more
economically prepared from p-methoxyphenol).
SeO2 is otherwise used for allylic oxidations but they report no isolation of such a product.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
Thank you very much Nicodem for this very thorough analysis. I had also some concerns about the picrate extraction and the isomerization/deoxygenation
versus reactional time. Too good to be true.
Since I have both compounds handy, I will run a few nanos and post my results because I am just a curious little fellow 
[Edited on 23-9-2008 by Quantum_Dom]
|
|
|
Quantum_Dom
Hazard to Self
 
Posts: 88
Registered: 23-6-2008
Member Is Offline
Mood: Entangled
|
|
| Quote: | Originally posted by Nicodem
such a reaction would obviously be of no preparative use as is now (especially considering that 2-5-dimethoxypropenylbenzene is much more economically
prepared from p-methoxyphenol). |
Thats for sure, its just that some amateur experimentalist dont happen to have allyl bromide handy everyday of the week  . .
|
|
|