Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Production of Ethene- destructive distillation of HD/LDPE
Today i decided to make some ethene (just for the hell of it  ) )
A bit of research gave me these methods:
1. Boil a mixture of Ethanol and conc sulphuric acid 1:2
Pass vapors through a soidum hydroxide solution to remove SO2 impuriity.
2. Boil Ethanol and lead vapours over a heated aluminium oxide catalyst.
3. Destructive distillation of HD/LDPE. gradually heat the PE until it melts and keep up the gradual heating until it releases Ethene.
I decided to go with number 3, Destructive distillation of HDPE as it seemed the easiest and cheapest ( you would have to use pure ethanol without
methanol to prevent CO forming)
Now, i had never heard of this method before, but this site claims it works:
http://mattson.creighton.edu/C2H4/index.html
For starters i added a small amount of cut up milk bottle (HDPE) to a test tube and began heating gradually, if you heat it too fast a white fog forms
( i think evaporating HDPE)
I heated the test tube slowly. wen it had melted i heated it slightly quicker and it bubbled giving off a gas. This gas was flammable and burnt with a
yellow flame. I bubbled it through a weak soloution of bromine in water (bromine water  ) but after 3 mins the water hadnt gone colourless :O ) but after 3 mins the water hadnt gone colourless :O
What could this gas be?
Thanks for any help, comments
Picric-A
|
|
|
chloric1
International Hazard
    
Posts: 1162
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
When it burned with a yellow flame, did it produce any black smoke or soot?
I think the bromine test has to be done in a nonpolar solvent like hexane. Got lighter fluid? When bromine is in water you may instead get
bromohydrin.
Fellow molecular manipulator
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
yep i got lighter fluid, is that hexane?
nope no soot, I will try using lighter fluid tommorox and see how that turns out.
Thanks,
Picric-A
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
PE does not nearly scission to ethylene, but rather to a mixture of alkanes and alkenes predominately in the C4 through C9 range. Styrenes and
acrylics do mostly convert to their monomers, most other plastics do not.
The site you reference is not giving yields, which I would expect to be low. You simple could have too much bromine for the amount of all alkenes
formed, a longer heating might have shown a change. Also, try a crystal or two of KMnO4 in water as a means of detection.
[Edited on 4-9-2008 by not_important]
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
Surly then when you cool the remaining gasses comming out at around -3 degrees C they will condense?
the gasses that came out from my decomposing ethene didnt condense...weird 
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I Have tried all of these methods and then some. I have found some to be much more practical than others.
1. Works, can be accelerated by certain metal salts; Phosphoric acid is better (so I have read); supposedly much less ether is produced also product
contains no SO2.
2. Works; But the catalyst must be heated to ~350 degrees (or around there depending on nature of catalyst). If you are working with alumina powder
you must disperse it onto a support (glass wool for me).
3. Is silly; I did it but the product is really impure; some of it even condensing as a white solid!
I have tried leading Naptha over strongly heated alumina dispersed on glass wool. Once the naptha was passed over the alumina started glowing a dull
red. The crude gas was passed through a basic solution to remove carbon dioxide and heavier hydrocarbons. I did not do any quantitative tests, but
it smelled like ethylene and it burned nicely sometimes with a nice 'thud' with proper air/olefin mixtures. Of course this product was likely
contaminated with other alkenes and hydrogen. I did it in a long test tube which was filled with 1.5ml alcohol then the catalyst was slid in. The
entire length of catalyst was gently heated with a torch until the ethanol at the bottom of the tube started boiling (allowing it to travel over the
catalyst); gas was collected in a pneumatic trough. It was quick easy and pretty fun.
*Other Methods*
Ethanol over heated pumice, silica or aluminum phosphate.
Ethanol over heated phosphoric acid dispersed onto coke catalyst (old industrial method).
Heating ethanol with an excess boric acid; decomposing the borate ester to form ethylene (never really worked that well for me).
From alkyl halides (wasteful)
[Edited on 9-3-2008 by smuv]
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
hey thanks for the great reply smuv!
My Al2O3 is actually a powder mixed with glass wool and i only heat it to around 150 degrees C but it seems to work fine. I absorb the ethanol in
mineral wool (cotton wool also works) and put it in the end of the test tube so you dont have vigerous bumping ethanol and you can put the test tube
horizontal.
Havnt heard of the Boric acid method...seems interesting, i will explore 
I havnt tried the aluminium phosphate method but i dont think i will seeing as it is easier to get pumice than it.
Can you use sand instead of silica? now that would be usefull :p
Thanks,
Picric-A
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Absolute alcohol can be dehydrated by mixing it with 4 times its weight of fused B2O3 and then gently heating in a retort, the ethylene forms and can
be made pure by a simple water wash as mentioned in the Dictionary of Applied Chemistry, 2, 566.
B2O3 can be made easily by roasting H3BO3 at high heat (e.g. with a torch) until the glassy solid shows (do this in a strong metal bowl or dish), let
it cool and then break it up.
Preferable over H2SO4, which also yields other nasties like small amounts of diethyl sulfate (peppermint odor) on heating the ethylsulfuric acid
(Beilstein I 332: diethyl sulfate from ethanol and H2SO4).
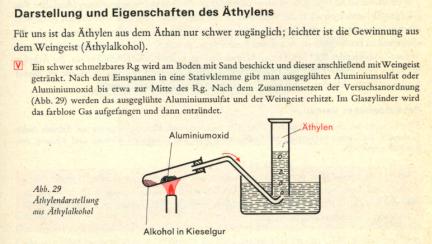
Below is an illustration from Organische Chemie by Braun, Krieger, 41: a heat-durable test tube has some sand added to it and then this is drenched
with ethanol. After securing this to a clamp, glowed aluminum sulfate or Al2O3 is put in about the middle of the glass. Then after setting it up as
shown, the heated aluminum sulfate and alcohol are heated. I would also start heating gently to drive out some air.
[Edited on 4-9-2008 by Schockwave]
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Wow @150C; this is really low does your product smell a lot like ether? Definitely at this temp some ethylene will form; I have never seen a
reference for good yields at such low temperatures (although it may be possible with a long residence time in the catalyst). What alumina did you
use? Commercial alumina from the bayer process is said to be an a pretty bad catalyst. I prepared mine by passing a stream of CO2 through a solution
of sodium aluminate. I then calcinated the aluminum hydroxide (after many washings to remove Na+) @ ~200c .
Use the search engine I there is another thread about ethylene which will probably be helpful. Sand will work as a catalyst; details are in the other
thread...it seems most metal oxides can produce some ether. The first time ethylene was synthesized catalytically in vapor phase a clay pipe was the
catalyst (google 'dutch chemists' as they did a lot of pioneering work with ethylene).
These are a really fun set of experiments; I hope you enjoy them as much as I.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
I bought myn form a chemical supplier, it mentioins being made by thermal dehydration of aluminium hydroxide.
I have read a good catalyst can be made by addition of Sodium hydroxide solution to acidic aluminium sulphate to precipitate the hydroxide followed by
roasting at 1050 degrees C to produce the oxide. My source:
http://www.chemeng.lth.se/exjobb/011.pdf
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
According to JACS 46, 390 (1924), the conversion with alumina is touchy, and 350C was the best temperature.
I've heard that the other preparations are fully detailed in books. Several of them.
BTW I've heard a chemistry professor at a major college liken the above link host, Creighton U., to an offshore island med school.
|
|
|
Ketone
Harmless

Posts: 17
Registered: 24-8-2008
Member Is Offline
Mood: Dipolar
|
|
| Quote: | Originally posted by chloric1
When it burned with a yellow flame, did it produce any black smoke or soot?
I think the bromine test has to be done in a nonpolar solvent like hexane. Got lighter fluid? When bromine is in water you may instead get
bromohydrin. |
Well, the solvent does affect what the product is..
Cause yeah, 2-Bromoethanol should be the main product instead of Dibromoethane if the Bromine is dissolved in water.
But isn't that colorless too?
My guess is that the gas wasn't Ethene. Or only v. little of it mixed with a huge load of other crap.
So I'd say go with method 1, it's tried and true and all that..
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
@S.C. Wack; In that paper the author uses very little catalyst (12cm long x 2cm diam); also his method of preparing the alumina catalyst is not
optimal (the history of the catalyst is very important). This was and still may be to a small extent an industrial process; if done correctly yields
and conversions per pass can be +90%.
@Picric-A Another way of preparing active catalysts is the precipitation aluminum hydroxide by boiling a dilute solution of aluminum chloride with a
large excess of urea. The only problem with this is the solution of AlCl3 must be very dilute require large reaction vessels to make reasonable
amounts (250g). I tried this on a test tube scale with more concentrated solutions and it (as expected) failed. I have seen refrences for the same
procedure applied to aluminum sulfate but I have no experience with this method.
While the method you posted probably works; I would first try one of the methods posted in J. Am. Chem. Soc. 47(11), 1925, pp 2748-2754. below as
these were extensively tested. While this article is for the production of ether; at higher temperatures these catalysts will also nicely produce
ethylene (at least one of them did for me).
BTW Nearly all commerical alumina is produced via the bayer process and is said to be a poor catalyst...I have no experience with bayer alumina for
this catalysis. I beleive the problem lies in high sodium contamination. I have seen patents outlining how to clean up bayer alumina, but I have no
practical experience with this process.
Some Refreances...
SCM Thread-Preparation of ethylene
The catalytic Preparation of ether from alcohol by means of aluminum oxide.
J. Am. Chem. Soc. 47(11), 1925, pp 2748-2754.
The catalytic Dehydration of ethanol and ether by Alumna
J. Am. Chem. Soc. 46, 1924, pp 390
Preparation of catalytically active gamma-Alumina...
Applied Cutalysis, 24 (1986) 25-35
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Picric-A
Surly then when you cool the remaining gasses comming out at around -3 degrees C they will condense?
the gasses that came out from my decomposing ethene didnt condense...weird 
|
-3 C won't do a go job condensing n-butane unless you had effective heat transfer, and will not condense iso-butane or the C1-C3 hydrocarbons.
LDPE has a fair amount of branching, thermal depolymerisation leads to some 'iso' hydrocarbons, which have lower boiling points than the straight
chain isomers.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
cool thanks, not_important, i will bear that in mind,
hmm maybe if you cool it in a dry ice/propanone bath that will be an effective/cheap way of purifying it..?
i think i will stick to the ethanol/Al2O3 method though, it seems a little less hasstle..
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Yup, the EtOH+hot tube is easier for getting decent yields. You'll likely get some diethyl ether and so may need to condense it out.
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
Just pass it through water; it is soluble enough to just dissolve in there; IIRC its solubility is ~5%. Efficiently condensing ether out will require
very cold temperatures much less than 34 degrees.
"Titanium tetrachloride…You sly temptress." --Walter Bishop
|
|
|
Saber
alias Picric-A
 
Posts: 56
Registered: 6-9-2008
Location: England
Member Is Offline
Mood: vry vry bad mood
|
|
I tryed this once, heating a mix of high and low density PE and condensing the vapors in a salt ice bath. i got a liquid that smelt rather of
propan-2-ol! after leaving the condensate in the test tube for a while i let it get to room temp to see if it would evaporate, and it did  any ideas what this was? any ideas what this was?
@Picric-A- i remember reading somewhere you get around 12% ethene, so infact if you find a way of removing other hydrocarbons it wouldnt be a bad
procedure.
|
|
|