Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
2-Methylbut-3-en-2-ol
This compound is found in the volatile oil of the hop plant (Humulus) & is also an insect pheromone. There are references to it having a
sedative/hypnotic effect when inhaled.
Structure: CH2=CHC(Me)2OH
Looking at the tert-alcohol structure, this stuff probably smells like camphor & is volatile (and flammable).
I couldn't find anything on its synthesis in a quick web & patent search but the possible routes incloude the following:
1. Addition of methyl Grignard reagent to methyl vinyl ketone. The latter can be made (and used promptly) from the Mannich base of acetone.
Structures: CH3C(O)CH2CH2NRR > CH3C(O)CH=CH2
I'd have to do a full lit sdearch on this to see if conjugate addition would be a problem here.
2. Addition of vinyl Grignard reagent to acetone.
3. Partial hydrogenation of 2-methylbut-3-yn-2-ol, an industrial chemical said to have narcotic effects itself and to be an irritant.
Structure: HC(triple bond)CC(Me)2OH
This last material is of interest as an acetylene derivative. Acetylene can be metallated & the metalloacetylene added to acetone.
| Quote: | Abstract of GB813490 Tertiary acetylenic alcohols are produced by reacting acetylene with a ketone under substantially anhydrous conditions and in the
presence of an alkali metal alkoxyethoxide of formula ROCH2CH2OM in solution in an alkoxyethanol of formula ROCH2CH2OH, where R in each case
represents an alkyl group of one to six carbon atoms, to form the alkali metal derivative of a tertiary acetylenic alcohol, and recovering the alcohol
from its alkali metal derivative. Preferably the alkali metal alkoxyethoxide is in solution in the corresponding alkoxyethanol, in which case the
solution may be prepared by adding aqueous alkali metal hydroxide to an excess of alkoxyethanol at a subatmospheric pressure, maintaining the mixture
at boiling point, and removing water by distillation with a hydrocarbon entraining agent, such as benzene or toluene. A preferred condensing agent is
potassium n-butoxyethoxide in n-butoxyethanol. Suitable ketones for carrying out the process are acetone, methyl ethyl ketone, methyl propyl ketone,
diethyl ketone, methyl amyl ketone, di-isopropyl ketone, cyclohexanone, and methylheptenone. The molar ratio of ketone to alkoxyethoxide is suitably
from 0.30: 1 to 0.95: 1, preferably about 0.75: 1. The condensation may be carried out at below 60 DEG C., preferably 0-30 DEG C. The pressure may be
atmospheric or superatmospheric. The tertiary acetylenic alcohol may be liberated from its alkali metal derivative by (i) adding water, extracting
with a solvent at a comparatively low temperature, and recovering the free alcohol from the solvent; (ii) hydrolysis, treating with CO2, removing
alkali metal carbonate, and solvent extraction; (iii) adding water, removing or neutralizing alkali metal hydroxide, and distilling; (iv) washing the
crude product with water, converting the alkali metal derivative to free alcohol, neutralizing alkali metal hydroxide and distilling; (v) (where
acetone or methyl ethyl ketone is used) distilling the crude reaction product, after adding water, at not above 60 DEG C. under reduced pressure, e.g.
100-150 mm., to obtain the free alcohol. In Examples (1) and (2) acetone is reacted with acetylene to produce 2-methyl-but-3-yn-2-ol;
(3), (4), (5) and (8) methyl ethyl ketone produces 3-methyl-pent-1-yn-3-ol; (6) and (7) 6-methylhept-5-ene-2-one produces dehydrolinalol (all using
potassium n-butoxyethoxide in n-butoxyethanol); and methyl ethyl ketone produces 3-methyl-pent-1-yn-3-ol, using (a) potassium n-propoxyethoxide in
n-propoxyethanol; and (10) sodium n-butoxyethoxide in n-butoxyethanol.
|
[Edited on 30-6-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
I think it would be better to react an acrylate with a methyl grignard reagent; Michael addition should be minimal.
Do you have any more info on its physiological effects? Do you know what its MAC is?
If you are after inhalants though, why not just make some ether? Stay away from chlorinated stuff though (hepatic toxicity etc). And if you are
after a hypnotic...barbiturates or methaqualone could be an interesting and not too out of reach project.
Your MSDS link isn't working for me, but I could see that substance being metabolized into an epoxide; for your sake I hope that I am wrong.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by smuv
I think it would be better to react an acrylate with a methyl grignard reagent; Michael addition should be minimal. |
Interesting option.
| Quote: | | Do you have any more info on its physiological effects? Do you know what its MAC is? |
Not off-hand.
| Quote: | If you are after inhalants though, why not just make some ether? Stay away from chlorinated stuff though (hepatic toxicity etc). And if you are
after a hypnotic...barbiturates or methaqualone could be an interesting and not too out of reach project.
|
I thought this reference was interesting. Ether is no piece of cake. The thought was alternatives to controlled substances.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
smuv
National Hazard
   
Posts: 842
Registered: 2-5-2007
Member Is Offline
Mood: Jingoistic
|
|
If you are just after obtaining it; and not the chemistry; Distill it from starting fluid. Although making ether is easy with the right glassware.
Granted if you have a jerry rigged system, its not as efficient, but you will certainly make ether.
Wash with/let the crude product stand over a strong NaOH solution, to remove SO2 and any other acidic crap as well as any acetaldehyde (cannizzaro
reaction) you may have made. At any rate ether is way easier to make than 2-methylbut-3-en-2-ol.
P.S. If ether is too commonplace for you, look into something like methoxypropane. A crossed ether should be fun to make (it is also physiologically
active, although with a more unplesant smell).
[Edited on 1-7-2008 by smuv]
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by smuv
If you are just after obtaining it; and not the chemistry; Distill it from starting fluid. Although making ether is easy with the right glassware.
Granted if you have a jerry rigged system, its not as efficient, but you will certainly make ether.
Wash with/let the crude product stand over a strong NaOH solution, to remove SO2 and any other acidic crap as well as any acetaldehyde (cannizzaro
reaction) you may have made. At any rate ether is way easier to make than 2-methylbut-3-en-2-ol.
P.S. If ether is too commonplace for you, look into something like methoxypropane. A crossed ether should be fun to make (it is also physiologically
active, although with a more unplesant smell).
[Edited on 1-7-2008 by smuv] |
May be, but I'm curious about molecules that are said to have interesting physiological effects but that are not controlled substances. I got my fill
of ether while working with it in college chem.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by smuv
If you are just after obtaining it; and not the chemistry; Distill it from starting fluid. Although making ether is easy with the right glassware.
Granted if you have a jerry rigged system, its not as efficient, but you will certainly make ether.
Wash with/let the crude product stand over a strong NaOH solution, to remove SO2 and any other acidic crap as well as any acetaldehyde (cannizzaro
reaction) you may have made. At any rate ether is way easier to make than 2-methylbut-3-en-2-ol.
P.S. If ether is too commonplace for you, look into something like methoxypropane. A crossed ether should be fun to make (it is also physiologically
active, although with a more unplesant smell).
[Edited on 1-7-2008 by smuv] |
May be, but I'm curious about molecules that are said to have interesting physiological effects but that are not controlled substances. I got my fill
of ether while working with it in college chem.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
2-methylbut-3-en-2-ol
| Quote: | Originally posted by Ritter
This compound is found in the volatile oil of the hop plant (Humulus) & is also an insect pheromone. There are references to it having a
sedative/hypnotic effect when inhaled.
Structure: CH2=CHC(Me)2OH
Looking at the tert-alcohol structure, this stuff probably smells like camphor & is volatile (and flammable).(cut) |
Being an unsaturated C5 compound, it would be classed as a semiterpene (more specifically a semiterpineol), unlike camphor, which is a C10 monoterpene
and also a ketone. Also, having the structure that it has as a tertiary alcohol, and also being a beta-enol rather than an alpha-enol, there is no
possibility of the compound undergoing non-structural keto-enol tautomerism.
Hop plants are grown extensively, for beer brewing, in the north of the South Island of New Zealand, especially around Nelson, and I could obtain hops
fairly cheaply from local brewers' supplies shops. I wonder if the stuff could be easily isolated from the plant's constituents, by either
distillation (if necessary with steam or under reduced pressure) or solvent extraction.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Looking at that tertiary alcohol, I can't but expect it to eliminate and go to the conjugated diene very readily.
I think the obvious ways to search for this compound's synthesis are by Chemical Abstracts (in a library, free, or online, expensive); Beilstein, same
choices, etc. IMO the patent literature is only likely to come up with answers if the target compound were of technological importance. Searching
the journalswould be hit and miss.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by JohnWW
2-methylbut-3-en-2-ol
Hop plants are grown extensively, for beer brewing, in the north of the South Island of New Zealand, especially around Nelson, and I could obtain hops
fairly cheaply from local brewers' supplies shops. I wonder if the stuff could be easily isolated from the plant's constituents, by either
distillation (if necessary with steam or under reduced pressure) or solvent extraction. |
I think you will have a problem here. The volatile oil fraction in hops is very small compared to the overall plant mass. And it contains other
compounds. You may have to process tons of hops to get enough oil to work with, then you have the separation & identification task. Sounds like a
lot of waste & work.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Abstract of GB813490 Tertiary acetylenic alcohols are produced by reacting acetylene with a ketone under substantially anhydrous conditions and in the
presence of an alkali metal alkoxyethoxide of formula ROCH2CH2OM in solution in an alkoxyethanol of formula ROCH2CH2OH, where R in each case
represents an alkyl group of one to six carbon atoms, to form the alkali metal derivative of a tertiary acetylenic alcohol, and recovering the alcohol
from its alkali metal derivative. Preferably the alkali metal alkoxyethoxide is in solution in the corresponding alkoxyethanol, in which case the
solution may be prepared by adding aqueous alkali metal hydroxide to an excess of alkoxyethanol at a subatmospheric pressure, maintaining the mixture
at boiling point, and removing water by distillation with a hydrocarbon entraining agent, such as benzene or toluene. A preferred condensing agent is
potassium n-butoxyethoxide in n-butoxyethanol. Suitable ketones for carrying out the process are acetone, methyl ethyl ketone, methyl propyl ketone,
diethyl ketone, methyl amyl ketone, di-isopropyl ketone, cyclohexanone, and methylheptenone. The molar ratio of ketone to alkoxyethoxide is suitably
from 0.30: 1 to 0.95: 1, preferably about 0.75: 1. The condensation may be carried out at below 60 DEG C., preferably 0-30 DEG C. The pressure may be
atmospheric or superatmospheric. The tertiary acetylenic alcohol may be liberated from its alkali metal derivative by (i) adding water, extracting
with a solvent at a comparatively low temperature, and recovering the free alcohol from the solvent; (ii) hydrolysis, treating with CO2, removing
alkali metal carbonate, and solvent extraction; (iii) adding water, removing or neutralizing alkali metal hydroxide, and distilling; (iv) washing the
crude product with water, converting the alkali metal derivative to free alcohol, neutralizing alkali metal hydroxide and distilling; (v) (where
acetone or methyl ethyl ketone is used) distilling the crude reaction product, after adding water, at not above 60 DEG C. under reduced pressure, e.g.
100-150 mm., to obtain the free alcohol. In Examples (1) and (2) acetone is reacted with acetylene to produce 2-methyl-but-3-yn-2-ol; (3), (4), (5)
and (8) methyl ethyl ketone produces 3-methyl-pent-1-yn-3-ol; (6) and (7) 6-methylhept-5-ene-2-one produces dehydrolinalol (all using
potassium n-butoxyethoxide in n-butoxyethanol); and methyl ethyl ketone produces 3-methyl-pent-1-yn-3-ol, using (a) potassium n-propoxyethoxide in
n-propoxyethanol; and (10) sodium n-butoxyethoxide in n-butoxyethanol.
|
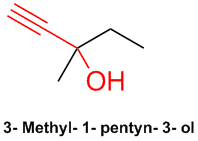
Meparfynol is 3-methyl-1-pentyn-3-ol, another liquid, acetylenic, tertiary carbinol that has been used as a sedative
& hypnotic. It was patented by Bayer in 1913. This is also commonly referred to as methylpentynol. Here is the Wiki with references:
http://en.wikipedia.org/wiki/Methylpentynol
[Edited on 28-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
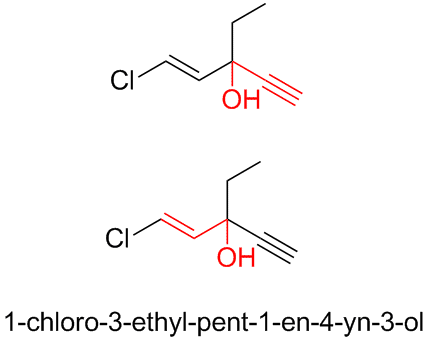
There is also the infamous ethchlorvinol, the sedative/hypnotic that caused Justice Rehnquist to hallucinate while recovering from surgery in 1971.
(http://en.wikipedia.org/wiki/Ethchlorvynol)
The ChemDraw shows that this molecule combines both the acetylenic tertiary carbinol structure with the allylic tertiary carbinol structure as well.
[Edited on 28-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Both those drugs described by Ritter are chiral, existing in two stereoisomers. In each case, are both stereoisomers physiologically active and are
supplied as racemic mixtures, or is only one of them of use as a drug?
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by JohnWW
Both those drugs described by Ritter are chiral, existing in two stereoisomers. In each case, are both stereoisomers physiologically active and are
supplied as racemic mixtures, or is only one of them of use as a drug? |
Since most drugs, chiral or not, produce their reaction at receptors, these unsaturated tertiary carbinols could be expected to show different
activities between their enantiomers. However, both are old & long discontinued (at least in the U.S). They were marketed as the racemates.
The brief searches I did in PubMed did not indicate any recent research in this area with these compounds.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Bluetooth
Harmless

Posts: 14
Registered: 19-8-2007
Member Is Offline
Mood: No Mood
|
|
I found a article on the toxic effects and side effects of methylpentynol right here:-
http://ukpmc.ac.uk/picrender.cgi?artid=1114359&blobtype=...
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Anyone who has had syrgery under one of the organo halide anaesthetics knows these aren't really much fun and feel pretty toxic. As an alternative to
controlled substances? I would say ether would be a lot less toxic and a, what, you can't buy diethyl ether in NZ? Making small amounts of ether in
the lab is not too daunting and the procedure is found in a number of 1st year Organic chem lab manuals. I blew the syntyhesis a few years ago but I
was drastically upscaling and also failed to watch the temperature closely enough. There was no question in my mind about the procedure.. I just
wasn't paying enough attention when I did it. I don't belive this is for the sake of making an inhalent. There are so many easy ones that are much
less toxic. Glue solvents for example, mix of toluene and pet ether? So what is this a precursor for? If you're willing to share the secret Ritter
old friend...
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
Globey
Hazard to Others
  
Posts: 183
Registered: 9-2-2009
Member Is Offline
Mood: No Mood
|
|
Have heard that diisopropylether has a very special effect which distinguishes itself amongst the huffies. Interestingly, halothane & friends
(R134a) are thought to interact with mU-opioid receptor, amongst other mechanisms. Ethanol defiantly does. Karl Marx was wrong about religion being
the "opiate of the people"...it was clearly wodka.
The one time I was put under general, I woke up crying my eyes out, and so angry, the nurse who kept "trying to help", but I just wanted to be left
alone. I sent her away almost crying. The two male anesthetists just ignored me (which is what I wanted). They were much more seasoned, and
probably knew someone in my state just wants to be left alone. Sort of like when you bump your head, and when someone says "oh, are you OK? Are you
OK???!?", and you just want to punch them...nothing personal. (end of ADD ramble)
|
|
|
cyclohexane
Harmless

Posts: 22
Registered: 5-1-2008
Location: The Evil Empire
Member Is Offline
Mood: happy
|
|
1. Addition of methyl Grignard reagent to methyl vinyl ketone. The latter can be made (and used promptly) from the Mannich base of acetone.
***sigma aldrich discontinued there production of this ( tho i believe it can be special ordered at greater cost...it was already overpriced in small
amounts)***
any info on its synthesis would be helpful. i assume this is a mannich condensation?
|
|
|