| Pages:
1
2 |
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
How would you go around synthesizing this molecule?
I want to experiment with molecules with a similar shape of those that are selective dopamine re-uptake inhibitors like GBR-12935. Since I don't have
a lab I figured that the closest molecule I could get from OCT chemicals is this one:
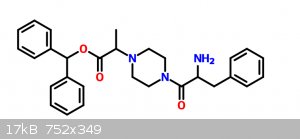
I'm planning on starting with Benzhydrol, alanine and phenylalanine... Most of the reactions are variations from quite common reactions and they
should be possible, my only obstacle is the piperazine ring formation. I was planing to make a chloramide derivative from the amide of phenylalanine
by the TCCA method and then close the ring with the amine group on alanine but I have never messed around with neither chloramines or chloramides so I
don't even know if this is possible.
Any suggestion will be greatly received! thanks in advance guys
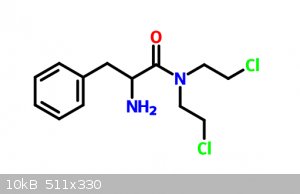 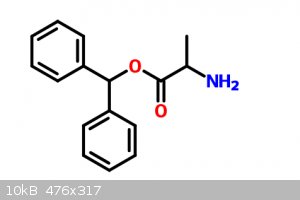
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
I would expect those two chlorides to react with the amino group on your phenylalanine molecule. You'll definitely have to figure out what to do
there.
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
What are your starting materials, those bottom molecules?
I imagine you could react the bottom left with the bottom right and get ring formation?
Start with DL-Phenylalanine, react that with urea (maybe) to get the corresponding amide. Then react it with a massive excess of 1-2-dichloroethane.
For the right prepare benzophenone, reduce it to an alcohol, then estify it with 2-amino-proponic acid.
This looks like a cool project, if you have all the reagents handy
You will need to protect that last amine for the final reaction.
The final reaction will be between those two to form that ring composed of the two tertiary amines.
[Edited on 19-11-2018 by VSEPR_VOID]
[Edited on 19-11-2018 by VSEPR_VOID]
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
My basic idea is:
For compound A (the right bottom molecule):
1.) Phenylalanine --(Phthalimide/Ferric Nitrate*)--> Phenylalanine-Phthalimide
2.) Phenylalanine-Phthalimide --(Urea/Boric acid catalyst)--> Phenylalanylamide-Phthalimide
3.) Phenylalanylamide-Phthalimide --(TCCA method/1,2-dichloroethane)--> Phenylalanylamide-Phthalimide N,N-bis(2-chloroethyl)
*DOI: 10.1021/jo500562w
For compound B (the left bottom one):
1.) Benzhydrol --chlorination/iodation--> Diphenylhalomethane
2.) Diphenylhalomethane --Sodium alanine--> Alanine Benzhydrolate
Final reaction:
Phenylalanylamide-Phthalimide N,N-bis(2-chloroethyl) + Alanine Benzhydrolate --(??????)--> Target molecule
Deprotection:
Under 20%HCl/4h reflux, KOH or hydrazine? (Probably not going to mess up with hydrazine tho)
Sorry for the made up names! 
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | 3.) Phenylalanylamide-Phthalimide --(TCCA method/1,2-dichloroethane)--> Phenylalanylamide-Phthalimide N,N-bis(2-chloroethyl)
|
I doubt there is any situation in which TCCA facilitates the N-alkylation of amides. Consider revising.
|
|
|
Melgar
Anti-Spam Agent
    
Posts: 2004
Registered: 23-2-2010
Location: Connecticut
Member Is Offline
Mood: Estrified
|
|
Would the sodium salt of alanine be sodium alanate? Or is it just MSG that works like that?
The first step in the process of learning something is admitting that you don't know it already.
I'm givin' the spam shields max power at full warp, but they just dinna have the power! We're gonna have to evacuate to new forum software!
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
True, I messed up there, got confused with amine chlorination... my bad.
Well this makes things more difficult, the obvious thing would be n-alkylation with dichloroethylamine hydrochloride but I'm not going to mess up with
potential chemical agents so what about first reacting the amide with diethanolamine which is way easier to obtain (80 bucks on ebay) and then
halogenating the diethanol? I think it's the safest route unless there's another one easier.
Has anyone here worked with n-chloroalkyl amides derivatives before? is it too messy to work with them?
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I made a 1-substituted 2-chloroethyl amide en-route to an oxazoline under Appel conditions (but RT, overnight). So besides reaction with the
neighboring amine it, at least theoretically, can also cyclisize onto the amide. I would choose a route starting from some piperazine, say monoboc
piperazine?
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
Is a protecting group out of the question?
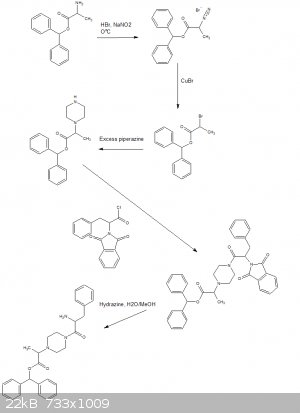
Sadly can think of no way to get around side alkylations of the undesired amines.
Phenylalanine could be reacted with phthalic anhydride then chlorinated to the acyl chloride with thionyl chloride or oxalyl chloride or phosphorous
pentachloride or any of your favorite chlorinating agents.
Someones gonna tell me this is over complicated 
Sufficiently advanced science is indistinguishable from madness.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
here is my method  - -
1.esterification of benzhydrol with 2-bromopropanoic acid and CDI -https://onlinelibrary.wiley.com/doi/abs/10.1002/978047084289...
2.https://www.jstage.jst.go.jp/article/cpb1958/41/1/41_1_148/_... ( the ref is given for isobutyl piperazine ,pg 8 in pdf)
3.https://pubs.rsc.org/en/content/articlelanding/2012/gc/c1gc1... ( to reduce coupling with the primary amine,the phenylalanine could be slowly
dripped into a pot containing the alkylated piperazine and CDI.The moment the COOH got activated,it would react preferentially with the excess
piperazine rather than another phenylalanine molecule  ) )
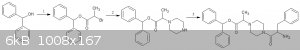
[Edited on 20-11-2018 by CuReUS]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Cureus, that was exactly what I was thinking. The only thing is that in the final step CDI is bound to react with the amine (piperazine or
phenylalanine) to form an imidazole urea. On phenyl alanine this may ringclose to form the carbamate, which just so happens to be a peptide coupling
reagent in itself (https://en.m.wikipedia.org/wiki/Bailey_peptide_synthesis https://en.m.wikipedia.org/wiki/Amino_acid_N-carboxyanhydrid...). So with just that minor adjustment that should be quite a consice route.
|
|
|
Assured Fish
Hazard to Others
  
Posts: 319
Registered: 31-8-2015
Location: Noo Z Land
Member Is Offline
Mood: Misanthropic
|
|
I missed the post where blackdragon stated using a phthalamide protecting group, i should read these threads more carefully.
| Quote: |
1.esterification of benzhydrol with 2-bromopropanoic acid and CDI
|
I dont understand why a normal fischer esterification cannot work here, the alanine will be protonated and would not likely be soluble in a non polar
solvent sure but using an excess of the benzhydrol to act as a solvent and sulfuric acid as catalyst should work fine, i see no reason to use exotic
reagents like CDI.
Sufficiently advanced science is indistinguishable from madness.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sigmatropic  | | The only thing is that in the final step CDI is bound to react with the amine (piperazine or phenylalanine) to form an imidazole urea. On phenyl
alanine this may ringclose to form the carbamate, which just so happens to be a peptide coupling reagent in itself |
Yes,you are correct.In that case, we should first slowly add phenylalanine to CDI.After everything has reacted,we add the
piperazine.
Alternatively,the NCA (4-Benzyloxazolidine-2,5-dione,CAS no-583-47-1) is directly available commercially.Reacting that with the piperazine would give
the target compound in 1 step without the need to use CDI.
the reason I suggested using CDI was because we would be using it anyway in the last step.This way we could carry out 2 reactions
with 1 reagent,making the route more efficient.Also benzhydrol is a sterically hindered alcohol,and I wanted to get a high yield of the ester as
quickly and cleanly as possible.But a fisher esterification could also be done. | Quote: | | the alanine will be protonated... |
we are using 2-bromopropanoic acid ,not alanine 
[Edited on 21-11-2018 by CuReUS]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Let's pretend you have SOCl2/triethylamine available.
phthalimido-phenylalanine [1. SOCl2; 2. phenol, triethylamine] >> phthalimido-phenylalanine phenyl ester
PPPE + piperazine >> mono-[phthalimido-phenylalanoyl]-piperazine
pyruvic acid + benzhydryl chloride + triethylamine >> benzhydryl pyruvate
benzhydryl pyruvate + mono-[phthalimido-phenylalanoyl]-piperazine + [reducing agent] >> phthalimido-GBR-12935
phthalimido-GBR-12935 + hydrazine >> GBR-12935
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
CuReUS Yeah I thought of that aswell, the problem is that I don't really have access to CDI... I'm only on a house-made lab, I don't have fancy
equipment or chemicals to be honest.
clearly_not_atara I've been trying to avoid working with potential chemical agents, SOCL2 is not really an option for me.
So I came up with another way I can realistically perform in my lab but I found another final obstacle:
For compound B:
1.) Benzhydrol --halogenation--> Diphenylhalomethane
2.) Diphenylhalomethane --Sodium alanine--> Alanine Benzhydrolate
3.) Alanine Benzhydrolate --NaNO2/HBr; CuBr (Sandmeyer)--> Bromo-Alanine Benzhydrolate
4.) Bromo-Alanine Benzhydrolate --Piperazine in acetonitrile/triethylammonium hydrochloride in acetonitrile*--> Alanine Benzhydrolate-Piperazine
*NOTE: Ref: http://www.arkat-usa.org/get-file/19416
For compound A:
1.) Phenylalanine --(Phthalimide/Ferric Nitrate)--> Phenylalanine-Phthalimide
2.) Phenylalanine-Phthalimide --???????????????--> N-Phthalimide-Phenylalanyl chloride
Final reaction would be similar for step 4 of compound B, Alanine Benzhydrolate-Piperazine with N-Phthalimide-Phenylalanyl chloride on
triethylammonium hydrochloride with acetonitrile as solvent.
Considering that for the acyl chloride I need to use thionyl chloride I don't really think I’ll be able to complete the final reaction... I thought
of maybe using PCl3 or maybe using a Boronic acid approach like so: Doi: 10.1016/S0040-4039(98)00503-6
This really escapes from my abilities and materials at my disposal.
I'll keep looking for a way to make the N-Phthalimide-Phenylalanyl chloride but if I can't find any I think I'll switch the reaction a little bit,
using the amine group on the phenylalanine is my first thought, halogenating it via Sandmeyer or hell-volhard-zelinsky (EDIT: don't mind this I got
confused) and then reacting it similarly as step 4 of compound B with triethylammonium hydrochloride on acetonitrile. Which is a pity because it would
had been interesting to study if the shape of phenylalanine on the molecule has neuronal activity and potentiates further dopaminergic release or
added noradrenergic release.
It would be something like this:
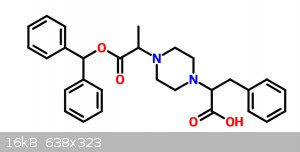
Another idea is to look for another starting material besides phenylalanine... my first idea is 3-amino-4-phenylbutanoic acid, protecting with
phthalimide/ferric nitrate, amidation with urea/boric acid and hofmann rearrangement to the amine, then it is pretty much straight forward to target
molecule (No idea how to get 3-amino-4-phenylbutanoic acid tho)
If anyone knows of a potential molecule that could be converted into 3-amino-4-phenylbutanoic acid or allows me to bypass acyl chloride I would be
eternally grateful to you my friend.
Thanks to everyone for your dedication and responses, they have been extremely helpful as they tend to be here on SM!
[Edited on 05/12/2013 by BlackDragon2712]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | 2.) Phenylalanine-Phthalimide --???????????????--> N-Phthalimide-Phenylalanyl chloride |
This step is the only reason that my suggested route used SOCl2. If you can form the acyl halide or similar of phtalimido-phenylalanine, it makes more
sense to convert this to a phenyl ester and react with piperazine, vs. trying to make a bis-chloroethyl amide. The reason is that
bis(chloroethyl)amine is far too dangerous to actually use, and N-alkylation of amides is nearly impossible. See:
https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/fi...
Alternatives include PBr3, POCl3, oxalyl chloride, cyanuric chloride, etc. Possibly the acyl tosylate formed with tosyl chloride could be used.
[Edited on 22-11-2018 by clearly_not_atara]
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by clearly_not_atara  | | Quote: | | 2.) Phenylalanine-Phthalimide --???????????????--> N-Phthalimide-Phenylalanyl chloride |
Alternatives include PBr3, POCl3, oxalyl chloride, cyanuric chloride, etc. Possibly the acyl tosylate formed with tosyl chloride could be used.
[Edited on 22-11-2018 by clearly_not_atara] |
Cyanuric chloride could work? 50 bucks for 250g huh? cool, now I guess it's about trying it out then.
I wonder if TCCA would also work... I only know of its use as a chlorinating agent of amines, doubt it tho.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
TCCA will not likely work as a substitute for cyanuric chloride for anything, because the environments that the chlorine atoms inhabit in these
compounds are entirely different. In TCCA, chlorine is in an oxidizing environment, in the +1 oxidation state, while in cyanuric chloride, it is in
the -1 oxidation state.
Substituting one for another is like a less extreme version of substituting an acid for a hydride. Sure they can both donate hydrogen, in a sense, but
their properties are very different, and switching one out for the other doesn't make sense.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by BlackDragon2712  |
1.) Benzhydrol --halogenation--> Diphenylhalomethane
2.) Diphenylhalomethane --Sodium alanine--> Alanine Benzhydrolate
3.) Alanine Benzhydrolate --NaNO2/HBr; CuBr (Sandmeyer)--> Bromo-Alanine Benzhydrolate |
I suggest you do
the sandmeyer on alanine first and then react it with benzhydrol,rather than after,since the sandmeyer is very messy and leads to a lot of crap
formation,which translates to a lower yield.Also there is no need to convert benzhydrol to halide,just do a direct fisher esterification with
2-bromopropionic acid. could you give a ref for
this step ?
generally the amine is reacted with phthalic anhydride to convert it to phthalimide.
wouldn't this step destroy the ester too ? 
| Quote: | | my first idea is 3-amino-4-phenylbutanoic acid, protecting with phthalimide/ferric nitrate, amidation with urea/boric acid and hofmann rearrangement
to the amine, then it is pretty much straight forward to target molecule |
I fail to see how this is straight
forward to the TM.After you carry out these steps ,there would be no COOH left to do the amidation reaction with piperazine.
| Quote: | | allows me to bypass acyl chloride |
One thing you could do would be to oxidise the NH2 of
phenylalanine to nitro using oxone,doing the amidation with piperazine ,and then reducing it back to amine using dithionite to get the TM.The nitro
might also activate the COOH enough to directly react with the piperazine 
[Edited on 22-11-2018 by CuReUS]
|
|
|
Cactuar
Harmless

Posts: 32
Registered: 25-7-2014
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
Say goodbye to the other amides and especially that ester.
|
|
|
BlackDragon2712
Hazard to Others
  
Posts: 124
Registered: 22-12-2012
Location: Everywhere
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by CuReUS  | I suggest you do the sandmeyer on alanine first and then react it with benzhydrol,rather than after,since the sandmeyer is very messy and leads to a
lot of crap formation,which translates to a lower yield.Also there is no need to convert benzhydrol to halide,just do a direct fisher esterification
with 2-bromopropionic acid. could you give a ref for
this step ?
generally the amine is reacted with phthalic anhydride to convert it to phthalimide.
[Edited on 22-11-2018 by CuReUS] |
Sure here: pubs.acs.org/doi/10.1021/jo500562w
I wont be doing the phthalimide protection tho, you guys are right, deprotecting it would be rather problematic
Yes it will, didn't think of that.
Quote: Originally posted by CuReUS  |
| Quote: | | my first idea is 3-amino-4-phenylbutanoic acid, protecting with phthalimide/ferric nitrate, amidation with urea/boric acid and hofmann rearrangement
to the amine, then it is pretty much straight forward to target molecule |
I fail to see how this is straight
forward to the TM.After you carry out these steps ,there would be no COOH left to do the amidation reaction with piperazine.
[Edited on 22-11-2018 by CuReUS] |
Yeah in there I was thinking on doing a sandmeyer and then reacting it with piperazine on a similar fashion as before with triethylamine on
acetonitrile, the other amine would had been protected
Quote: Originally posted by CuReUS  |
| Quote: | | allows me to bypass acyl chloride |
One thing you could do would be to oxidise the NH2 of
phenylalanine to nitro using oxone,doing the amidation with piperazine ,and then reducing it back to amine using dithionite to get the TM.The nitro
might also activate the COOH enough to directly react with the piperazine 
[Edited on 22-11-2018 by CuReUS] |
I like this idea... This is what I'm going to try so far:
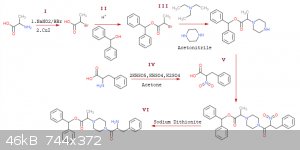
This really looks extremely doable and fun, I'm getting exiting over this! thanks CuReUS for your corrections and suggestions, extremely helpful!
EDIT: on reaction (I) I wrote CuI, I meant CuBr my bad... Also, is it a good idea to do the sandmeyer with HI and CuI instead of bromine? since iodide
is a better leaving group than bromide it should react easier with piperazine right? also workup would be simpler I think.
[Edited on 05/12/2013 by BlackDragon2712]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
 I didn't think about that very long, did I? I didn't think about that very long, did I?
Unfortunately, BOC is out too as a protecting group. Hydrogenolysis of BOC will also cleave the benzhydryl ester. There are some photolabile
protecting groups that could work (including ortho-nitrobenzyloxycarbonyl and phenacyloxycarbonyl), although you now have to work in the dark!
| Quote: | | One thing you could do would be to oxidise the NH2 of phenylalanine to nitro using oxone,doing the amidation with piperazine ,and then reducing it
back to amine using dithionite to get the TM.The nitro might also activate the COOH enough to directly react with the piperazine
|
As far as I'm aware the simple version with Oxone is limited to non-enolizeable amines. There are some versions that use e.g. dimethyldioxirane or
Zr(OtBu)4 etc, but most are involved.
A more practical procedure might be to perform the Sandmeyer reaction on phenylalanine to make the alpha-chlorophenylalanine, which forms the
nitroacid with sodium nitrite. I'm a bit unsure about the stability of nitroacids... it seems like unexpected condensations and rearrangements are a
possibility. The CH acidity of the alpha proton will be large.
|
|
|
Cactuar
Harmless

Posts: 32
Registered: 25-7-2014
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
I think step 3 will be difficult. The Et3N could E2 the bromide to an acryl ester. Too little piperazine and you might end up with a dimer, too much
and you will probably cleave the ester.
I would use MeCN like you suggested to promote Sn2 and I would slowly drop 1 eq ester into 1 eq piperazine in MeCN at 0 C and not letting it go above
0 C. Hoping that the HBr formed would quench the second amine on the piperazine which would minimize side reactions. The low temperature to minimize
elimination and ester cleavage. Maybe stir for 1h at 0C and then let it slowly reach RT and check progress on TLC. Then if necessary add small amounts
of Et3N with checking reaction progress repeatedly.
How will you do step 5?
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by BlackDragon2712  | | is it a good idea to do the sandmeyer with HI and CuI instead of bromine? since iodide is a better leaving group than bromide it should react easier
with piperazine right? also workup would be simpler I think. |
You don't need Cu for
iodination.NaNO2/HCl followed by KI works fine.Also the yield of sandmeyer is almost quantitative for iodination http://www.prepchem.com/synthesis-of-iodobenzene/
lastly,I2 is easier to handle than Br2 
As for the better leaving group idea,you are correct in assuming that it would react faster with the piperazine.Generally,the reason why
Br2 is preferred over I2 is due to cost.That's why Br2 is also called "poor man's I2" 
[Edited on 27-11-2018 by CuReUS]
|
|
|
Cactuar
Harmless

Posts: 32
Registered: 25-7-2014
Location: Denmark
Member Is Offline
Mood: No Mood
|
|
I don't really know anything about a-nitro carboxylic acids but doubt the amine and acid would condense. IFAIK that only happens intramolecularly. And
if the nitro group destabilize the acid that much, wouldn't it also very easily decarboxylate?
It would be worth a try but I think using a metyl ester or some peptide synthesis would be more successful.
|
|
|
| Pages:
1
2 |