| Pages:
1
2 |
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
Sodium Metal from NaCl Reduction using Lead
Fairly explicit title. I intend to use the process outlined in British Patent 23, 689; Vournasos, A.C. 1908 which involves heating lead metal and NaCl
to red heat, at which point the sodium distills over. Lead should be left as PbCl2. What would be the best method for recovering the lead metal:
Electrolysis with lead anode and graphite cathode? (Perhaps I can use lead/tin solder for the anode, thereby allowing the recovery process to be a
refining process as well.) The only problem might be the solubility of Lead Chloride. Are they any other practical methods for reducing the chloride
to the metal?
Another process I may attempt is the reduction of Sodium Tetraborate or sodium bicarbonate with carbon. The drawbacks for this is that copious amounts
of CO are produced. Perhaps I'll conduct it into a chamber with scrap nickel to make some "Death Juice."
Yet another considered is carbon reduction of NaCl using CaO. This would likely be the most inconvenient as it produces just enough CO to be annoying,
and would require some method to recover CaO. I would likely rather use a Downs Cell, which I will probably end up doing anyway. (Now if I can get
around to building a stirling cryocooler to condense that Cl2.) Or perhaps the gas liquefier posted previously on these forums [somewhere,] though I'm
hesitant to put Cl2 though a compressor.
Well then, enough rambling! Discussion: Production of Sodium Metal using non electrochemical methods. (Preferably via the Lead reduction/recovery
process, as the cycle is near fully regenerative.)
[Edited on 5-18-2008 by ShadowWarrior4444]
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
You can electrolyse molten lead (II) bromide mp 367°C to recover lead (molten) and bromine (vapourises off obviously) - this was a demonstration of
non-aqueous elcetrolysis in school chemistry class! Perhaps lead (II) chloride mp 501°C could be easily melted with a burner and electrolysed to
recover your lead? Carbon anodes would be used but chlorine at that temperature would likely eat the carbon quickly so replacement would be needed.
There is a post on unconventional routes to sodium somewhere in this forum already but I hope the above will help you.
Good luck! 
p.s. obvious but distilation of sodium must be done in inert atmosphere such as argon.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
ShadowWarrior4444, check out excellent papers scanned by BromicAcid in his page: http://members.aol.com/bromicacid/sodium/ or go to Tacho's page for an related experiment: http://www.tacho.kit.net/pag5.htm
Also like panziandi said, go to search and find out this thread:http://www.sciencemadness.org/talk/viewthread.php?tid=2105&page=1 or search other
thread..IIRC is another very extensive thread on sodium making, as well an excellent work posted on prepublications (by len1)..
Also, IIRC there are numerous other routes like lead + NaCl , some of them react to give sodium at even lower temps and all are , at least, briefly
described on Bromic page above.. have fun!
By the way, IMHO will be somewhat hard to recover your Pb after converting it to PbCl2.. But will you really need that? lead is usually very cheap and
even free from some sources..
Besides you can convert the lead chloride in other lead compounds more reactive (e.g. lead carbonate) that you can have just lying around to make
other Pb compounds (e.g. lead nitrate).
[Edited on 18-5-2008 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I agree that it would definitely not be worth it to recycle the lead. I would also use it to make other lead compounds or just keep it as is! I
think you have a pretty massive undertaking here. More so than you think. Especially the requirement of an inert atmosphere.
[Edited on 5-18-2008 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
I would first like to state that I have indeed read both Bromic's site, and the unconventional sodium threads. I have also searched the entire forum
using Google, and the find feature on the printable version of nearly every sodium thread.
As for the inert atmosphere requirement, it is quite a simple undertaking should I construct a full-scale reaction chamber; though, I have been
considering that it may be possible to produce sodium metal in its molten form simply by adding NaCl to molten lead. The molten sodium may be decanted
off the PbCl2, as I doubt it is soluble in molten sodium.
| Quote: | | Besides you can convert the lead chloride in other lead compounds more reactive (e.g. lead carbonate) that you can have just lying around to make
other Pb compounds (e.g. lead nitrate). |
Would anyone happen to know of a way to convert PbCl2 to other lead compounds that does not use an alkali nitrate?
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Hey,
Sodium is soluble in lead. In fact an alloy of sodium and lead can be used as a drying agent for some solvents instead of using neat sodium! Its a bit
like the amalgam.
I expect the red heat is vital in that lead is a poor reducer and the whole reaction would be driven by the fact the sodium is distilled out.
One thing I am looking into (not for sodium mind as I have an plentiful stock!) is the electrolysis of certain metal salts in organic solvents. I had
to put it on hold for the time being but will get back to it soon and will post my results on my website and of course on this forum!
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I find that hard to believe. Pb is quite unreactive, and its chloride quite volatile. Nascent Na would surely reduce it to Pb.
SW4444: CO burns readily in air.
Tim
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I have the same feeling as 12AX7. I would even expect it the other way around. If you mix PbCl2 and Na-metal, then I expect formation of NaCl and Pb.
If making Na from lead-metal and a sodium salt does work, then I am very interested in an explanation for this.
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | | Originally posted by ShadowWarrior4444 Another process I may attempt is the reduction of Sodium Tetraborate or sodium bicarbonate with
carbon. The drawbacks for this is that copious amounts of CO are produced. Perhaps I'll conduct it into a chamber with scrap nickel to make some
"Death Juice." |
Heating bicarbonate or carbonate of potassium or sodium with carbon is risky, because it can form an explosive by the combination of e.g. hot
potassium (or analogous sodium) metal with carbon monoxide to give the black potassium rhodizonate (K2C6O6), this compound explodes spontaneously when
dry and reacts violently with water. I've thought about roasting carbonates and carbon in pipes, but it sounds too risky.
[Edited on 19-5-2008 by Schockwave]
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by woelen
I have the same feeling as 12AX7. I would even expect it the other way around. If you mix PbCl2 and Na-metal, then I expect formation of NaCl and Pb.
If making Na from lead-metal and a sodium salt does work, then I am very interested in an explanation for this. |
It may be that the reaction is driven by the evaporation of sodium as previously mentioned. I also seem to recall that PbCl2 holds on to its chlorine
quite tightly.
Salient reference: http://members.aol.com/bromicacid/sodium/4.jpg
| Quote: | Originally posted by Schockwave
Heating bicarbonate or carbonate of potassium or sodium with carbon is risky, because it can form an explosive by the combination of e.g. hot
potassium (or analogous sodium) metal with carbon monoxide to give the black potassium rhodizonate (K2C6O6), this compound explodes spontaneously when dry and reacts violently with water. I've thought about roasting carbonates and carbon in
pipes, but it sounds too risky. |
I am immortal, infallible, and fearless. *smirk*
Also:
http://www.sciencelab.com/xMSDS-Rhodizonic_acid_dipotassium_...
http://msds.chem.ox.ac.uk/RH/rhodizonic_acid_dipotassium_sal...
In addition, Rhodizonic acid is used in Gun Shot Residue tests to detect lead. Rhodizonates should also decompose at around 300C, so a reaction
carried out above that temperature would not allow them to form.
As for the viability of the lead reaction, it seems that Lead Chloride boils at 950C whereas Sodium boils at 881.4C; were the reaction to be conducted
between those temperatures, sodium should distill smoothly.
[Edited on 5-19-2008 by ShadowWarrior4444]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
The MSDS aren't very useful, you have to look here, here, or here. Phew. Explosions have also result from trying to get potassium this way, you can read about this here.
[Edited on 19-5-2008 by Schockwave]
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
potassium carbonyl forms if the potassium vapour condenses in the presence of carbon monoxide. potssium carbonyl is given the structure of the
hexa-potassium salt of hexahydroxybenzene. di-potassium rhizonate is a different compound.
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by Schockwave
The MSDS aren't very useful, you have to look here, here, or here. Phew. Explosions have also result from trying to get potassium this way, you can read about this here, they call it the potassium salt of hexaoxybenzene.
[Edited on 19-5-2008 by Schockwave] |
I should note, that were I trying to obtain potassium by reduction of its carbonate, I would be using elemental silicon, not carbon. That said, these
reports of explosions are quite sketchy with some being attributed to the formation of potassium peroxide and its interaction with elemental carbon. I
don't anticipate any such unsavory occurrences while producing sodium from the carbonate method.
Ancillary reference: PROCESS FOR THE PREPARATION OF HEXAHYDROXYBENZENE
[Edited on 5-19-2008 by ShadowWarrior4444]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
This will clear it up:
Carbonyl potassium is KCO, it is different than hexahydroxybenzene potassium K6C6O6. Both of which are actually different than potassium rhodizonate
(K2C6O6). Then there is also potassium acetylenediolate (K.OC.:CO.K).
Hexahydroxybenzene potassium is made as the Lehrbuch der organischen Chemie by Victor Meyer describes by leading dry CO into molten potassium, it is a
grey mass and becomes highly explosive upon standing in air (they say it's harmless freshly prepared), this comprises the most significant part of the
black mass which had resulted in large explosions by the reduction to potassium, they say its formation can be completley avoided nowadays, but don't
give the details afterwards. Though at higher temperatures (above 180ºC) the reaction between CO and potassium mainly the hexahydroxybenzene compound
forms, below that near the m.p. of potassium around 62.3ºC acetylenediolate and an "organometallic compound" predominate. According to Meyer, potassium rhodizonate forms from the hexahydroxybenzene by washing it continuously with
dilute alcohol where it results by oxidation. Carbonyl potassium is described by Gmelin and is made by leading CO at -50ºC into a solution of K in
NH3, until it turns from blue to white-pink. It detonates at 100ºC, upon exposure to air or a drop of water it forms K2CO3, potassium oxide, and
carbon. Sorry about the confusion, I didn't know so many different compounds form from CO and potassium.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Very interesting info Schockwave.
As for why this works, all reduction-oxidation potentials are in equilibrium, and while the electrical potentials do indicate that nothing should
happen, as the equilibrium constant that can be calculated from the electrical potentials hugely favours lead and salt, keep in mind that since it is
in equilibrium, a small ammount of sodium will be present, which can be continously distilled off shifting the equilibrium to obtain more Na.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I still don't buy it. The boiling point of PbCl2 is very close to sodium's. If the reaction proceeds at all, they would be co-present in the
metallic (Pb, Na), salt (NaCl, PbCl2) and gaseous (PbCl2, Na) phases!
I would find Pb + NaOH more believable, as PbO has a somewhat higher melting and boiling point, but still nothing impressive, not like Al or Mg
reduction.
Besides, if this reaction proceeds, why not with somewhat more reactive and much cheaper Fe? FeCl2 would be the product, which has a sufficiently
high boiling point. FeO, Fe3O4 and Fe2O3 all have much higher melting and boiling points, yet there is no reaction between NaCl or NaOH and Fe.
Tim
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by 12AX7
I still don't buy it. The boiling point of PbCl2 is very close to sodium's. If the reaction proceeds at all, they would be co-present in the
metallic (Pb, Na), salt (NaCl, PbCl2) and gaseous (PbCl2, Na) phases!
I would find Pb + NaOH more believable, as PbO has a somewhat higher melting and boiling point, but still nothing impressive, not like Al or Mg
reduction.
Besides, if this reaction proceeds, why not with somewhat more reactive and much cheaper Fe? FeCl2 would be the product, which has a sufficiently
high boiling point. FeO, Fe3O4 and Fe2O3 all have much higher melting and boiling points, yet there is no reaction between NaCl or NaOH and Fe.
Tim |
There is a process that involves heating Iron and Sodium Fluoride under vacuum to obtain sodium; apparently it does not require either to melt. It
cites the high volatility of iron halides as the reason it cannot be used with NaCl. It is also mentioned that iron displaces sodium from many other
sodium compounds when heated under vacuum.
Hackspill, L. and Grandadam, R., Compt. Rend., 180, 68-70 (1925).
Soc. D'Electro Chimie, D'Electro-Metallurgie et des Acieres Electriques D'Ugine, French Patent 603,825 (1924).
Co-presence in the metallic phase to be expected, sodium is soluble in molten lead, and this solubility is exploited in many sodium production systems
to remove the sodium; it can be easily distilled out of the lead. I also suspect that this is one of the principles behind the NaCl reduction--some
sodium is formed, is absorbed into the lead, and is distilled out. It indicates that the lead is heated until just below red heat, at which point
sodium distills over.
I suppose the easiest way to settle this is attempting it on a small scale in a crucible. One doesn’t need to collect the sodium, simply watch to
the sodium oxide fumes.
[Edited on 5-19-2008 by ShadowWarrior4444]
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
Perhaps a more practical version of this Pb/NaCl would be to electrolyse a molten sodium salt with a molten lead cathode? Sodium would dissolve in the
molten lead forming an alloy, the sodium can then be distilled out of the alloy once you have accumulated enough? A eutectic of sodium salts being
used to allow operation at a "reasonable temperature"??? The lead cathode would be under the molten electrolyte and so the sodium wouldn't be attacked
by the atmospher or the by products of the reaction. Later the sodium could be distilled (perhaps a short path all-in-one setup under argon, the
condensed liquid run directly under paraffin liquid for solidification and storage?
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I suppose the advantage to that over, say, a Downs cell is, the sodium stays at the bottom, with lead? Might be a little easier.
Tim
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
No one can bother to actually read the patent before commenting unless there is a link, maybe even if there is.
http://v3.espacenet.com/origdoc?DB=EPODOC&IDX=GB19082368...
|
|
|
panziandi
Hazard to Others
  
Posts: 490
Registered: 3-10-2006
Location: UK
Member Is Offline
Mood: Bored
|
|
| Quote: | Originally posted by 12AX7
I suppose the advantage to that over, say, a Downs cell is, the sodium stays at the bottom, with lead? Might be a little easier.
Tim |
Yes quite the sodium dissolves in the lead and stay at the bottom of the melt. You wouldn't use NaOH as it reacts with lead, but a eutectic of halides
perhaps would work, IIRC a mix of sodium and calcium chlorides has a lower mp.
I didn't deny the validity of the patent. It may occur or it may not (like many patented experiments), patents never reveal the details or are peer
reviewed. However I merely suggested a modification.
SW4444:
I would try this reaction firstly in a cruicible like they state and see if the fully cooled melt does indeed show alkaline reaction with water. If it
does use a distillation method 2nd time around and try to isolate sodium. But do consider the electrolysis using a molted lead cathode, since I think
that is a promising variation of the patent.

|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
what temps do you need to heat the mixes ( of say, NaOH/Pb ) to?
surely it would be difficult to find a vessel able to take this heat and then find an attachment to connect a condenser?
|
|
|
ShadowWarrior4444
Hazard to Others
  
Posts: 226
Registered: 25-4-2008
Member Is Offline
Mood: Sunlight on a pure white wall.
|
|
| Quote: | Originally posted by Picric-A
what temps do you need to heat the mixes ( of say, NaOH/Pb ) to?
surely it would be difficult to find a vessel able to take this heat and then find an attachment to connect a condenser? |
The boiling point of sodium is 883C, this is *easily* attainable in a flower pot. Good quality ceramic crucibles are generally rated up to 1400C.
Incidentally, I do not believe you can use NaOH for a reduction with another metal; I seem to recall that it must be a non-volatile halide.
As for a condenser, a nice stainless steel pipe would be very useful, even fused quartz or perhaps with some creativity a borosilicate chamber could
be used. The main difficulty in condensing sodium is the inert atmosphere. (Regulating pressure and the like.)
|
|
|
tumadre
Hazard to Others
  
Posts: 172
Registered: 10-5-2005
Member Is Offline
Mood: No Mood
|
|
Salt will saturate a flower pot and corrode electrical heating elements, so make sure its physically separated or use gas.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
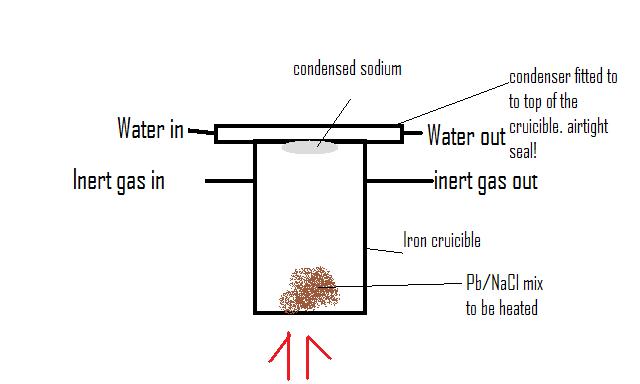
ok this is a pretty crap drawing but it pretty much shows what i am thinking.
The condenser on top be attached to the cruicible somehow to make an airtight seal and will have water flowin through it fast to cool down the
distilled sodium.
The cruicible will have two 'inlet and outlet' taps either side to flush out with inert gas (eg. helium which can be bought form party supply stores)
but then sealed when the distilling takes place so the sodium vapour doesnt get carried away.
|
|
|
| Pages:
1
2 |