Illegal Parkinson
Hazard to Self
 
Posts: 83
Registered: 2-10-2005
Member Is Offline
Mood: No Mood
|
|
4-Benzylpiperidine
Reductive amination between piperidine & benzaldehyde purportedly gives a ~96% yield of 4-Benzylpiperidine. This is somewhat surprising in that
one would expect the reduction of the Schiff base to give the N-substituted tertiary amine.
Alinezhad, Heshmatollah; Tajbakhsh, Mahmood; Salehian, Fatemeh (2005). "Reductive Amination of Aldehydes and Ketones to Their Corresponding Amines
with NaBH4 in Micellar Media". Monatshefte für Chemie - Chemical Monthly. 136 (12): 2029–2033. doi:10.1007/s00706-005-0362-3.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Must be some sort of typo, or otherwise it would be a very peculiar C(sp3) - H activation. Given that the paper is Iranian and the fact that it
features Micellar Media as a means of publishing something completely unoriginal I wouldn't believe the claims made. But perhaps these sort of things
happen when you run your reactions using mayonnaise as the solvent.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
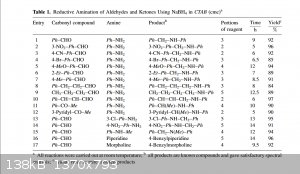
If you look at the paper, the result for "4-benzylpiperidine" is right next to the entry for "4-benzylmorpholine" where the latter actually indicates
N-alkylation. The authors do not comment on any unexpected results with piperidine. Possibly, whoever wrote up the article (probably a grad student)
mistakenly thought that piperidine was numbered like morpholine with the N at 4.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
12,13 and 14 h reactions? Come on now, one does that sort of thing for cell based experiments but not chemical reactions...
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
Quote: Originally posted by Sigmatropic  | | 12,13 and 14 h reactions? Come on now, one does that sort of thing for cell based experiments but not chemical reactions... |
I'm not sure where you pulled that idea from...
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Surely starting a reaction at 0800, only to finish it at 2200 is not what anyone who actually does this day in day out would do, don't you think?
Maybe it is different in your country but I don't consider that normal working hours.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It's quite common to leave a reaction running overnight (say, 20:00 to 8:00 the next day).
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I don't consider 8 to 8 normal working hours but that'll depend on your location.
A 14 hour reaction with 5 portions of reagent is not the same as setting up a reaction and coming back the next day.
Now I will leave this discussion and keep these thoughts to myself next time.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I hear about such reaction-times often in my research lab. Certainly for batch mode gas reactors like what I use, but the organic chemists also run
22hr, 14hr, etc. reactions and talk about them being pains. Often multiple grad students will be watching a reaction over its course.
|
|
|