| Pages:
1
..
7
8
9
10
11
..
77 |
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Looks like caramel to me.
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
It´s vanadinite 
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|

3,2,1, bang!
A gram of hexanitromannite initiated with some lead styphnate photographed just when it exploded.
The experiment was part of chemistry safety classes when the students learnt about how to handle explosives and other compounds. The audience was
protected by a plexiglass shield and noone got hurt, besides the plastic bottle with full of water what was next to the explosive.
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Wow, kristofvagyok , that looks great.
Not very pretty, but I just found a nice length of copper power wire on my street. I haven't taken off the insulation, but the whole chunk weighs 470
grams. I'm assuming that ~80% of it is copper, so I might get about 376 g's from it.

|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Zyklonb  | Wow, kristofvagyok , that looks great.
Not very pretty, but I just found a nice length of copper power wire on my street. I haven't taken off the insulation, but the whole chunk weighs 470
grams. I'm assuming that ~80% of it is copper, so I might get about 376 g's from it.
|
The copper used for electrical wire is very pure, and the insulation probably doesn't weigh very much.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I know, it's an excellent source. I wonder where it came from, there were no power line near where I found it. Especially no broken lines. Copper
thieves dropped it perhaps.... Probably not. Probably not.
I used to have to strip other electrical wire lying around for my copper, but this will last a long time.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
I found a similar piece of wire in an empty lot near my house, except it was made of aluminum. It should also be very pure! My neighborhood has a lot
of construction going on, so I'm always hunting around the sites for scraps 
EDIT: Hegi, I've never seen vanadinite that color before - it's always orange or red. Do you know why yours is different?
[Edited on 3-18-2014 by MrHomeScientist]
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|


It's just some small scale Iodine extraction, not that fantastic or anything but I like the colours xD
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
I built a little electroplating set up recently. thought I'd share as it is cheap and easy to make. haven't done a whole lot of work with it yet, but
it's currently refining sterling scrap.
main components:
1- DC-DC stepdown transformer, http://www.ebay.com/itm/LM2596-DC-DC-Buck-Converter-Constant...
1- DC digital ammeter/voltmeter, http://www.ebay.com/itm/141164937042?ssPageName=STRK:MEWNX:I...
and some simple stuff,.. A shallow light switch box( 1.25$), piece of plexiglass, a small piece of terminal strip, some wire and a stand to attach it
to... and a wine cork( generic non-conductive backing for adj. step-down board positioning)
    
pic 1) laid out the parts: stand, switch box, plexi and the new V/A meter
pic 2) assembled unit
pic 3) attached to the stand.
pic 4) side view, short foot on the display side, and long foot opposed it rests nicely under the plating tank to secure the stand from falling.
pic 5) action shot on initial run. in this one you can see the stainless spill tray, the stand and control unit with the silver cell peaking out
behind it( in a plastic bowl for shock/spill hazard) a small glass bowl to set sterling electrode while looking, and a second plastic cover to keep
debris out while cell is down.
the DC-DC stepdown unit is adjustable, and the adj. screws are set in the plexi face plate. it can be powered by a wall-wort( AC adapter > 5V),
and tuned down within a given range of the supply voltage. the amperage control isn't that great. it floats up/down quickly with voltage adj. but
your amperage adj. by itself has a short leash.
total cost to me ~15$ and a few hours works, as I already had spare project materials around. the actual cell is a single wall stainless steel travel
coffee mug with handle and all plastic taken off. the lid is some sort of PVC gutter/drain pipe cover( ~1$ ace ). any tighter and the lid wouldn't
fit. I'll have to put a few days through it and see how she handles, hopefully well.
plexiglass is a bitch to work with some times. or mine was just really brittle. I broke 2 pieces making the face plate. that was probably the
single most time consuming aspect.
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by MrHomeScientist  | I found a similar piece of wire in an empty lot near my house, except it was made of aluminum. It should also be very pure! My neighborhood has a lot
of construction going on, so I'm always hunting around the sites for scraps 
EDIT: Hegi, I've never seen vanadinite that color before - it's always orange or red. Do you know why yours is different?
[Edited on 3-18-2014 by MrHomeScientist] |
I have no idea... I did not study it... :/
Potassium ferrioxalate crystals

And a little bit of analytical chemistry. Complexometric titration of Cu2+

Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Quote: Originally posted by violet sin  | | I built a little electroplating set up recently. thought I'd share as it is cheap and easy to make. haven't done a whole lot of work with it yet, but
it's currently refining sterling scrap. |
Love it, hopefully I see a lot of work come out of that apparatus  There is such
a low barrier to entry for electrochemisty and a vast number of experiments to be performed, I wish I saw more of it on this forum. There is such
a low barrier to entry for electrochemisty and a vast number of experiments to be performed, I wish I saw more of it on this forum.
|
|
|
Töilet Plünger
Hazard to Self
 
Posts: 66
Registered: 27-2-2014
Location: /root/
Member Is Offline
Mood: No Mood
|
|
Hegi, what reagents were used in the titration? And you should take pictures of the ferrioxalate in UV as well!
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Definitely should but do not have UV right now... chelaton III as titrant and murexide as indicator were used 
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Potassium nitrate crystals freshly decanted from their solution. I really liked how they formed this starburst pattern in the upper part of the
beaker!

|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Came across a time lapse video (done on an iphone) of the Belousov-Zhabotinsky reaction in a petri dish using ferroin. Its not crazy good but the
effect is neat.
https://www.youtube.com/watch?v=qFgnDs9GZj0
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
By "came across", you mean "uploaded"?
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Came across a video I uploaded a long time ago while looking at my YouTube account  is what I should have said. I plan on making a nicer one now that I have a better camera and apps for this type of filming. I have a few indicator
mixes if like to try as well including a fluorescent one, so, we shall see how that works out is what I should have said. I plan on making a nicer one now that I have a better camera and apps for this type of filming. I have a few indicator
mixes if like to try as well including a fluorescent one, so, we shall see how that works out
[Edited on 29-3-2014 by Mailinmypocket]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
O-alkyl dithiocarbonates
 
[Edited on 29-3-2014 by Cheddite Cheese]
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Just some chromium compounds
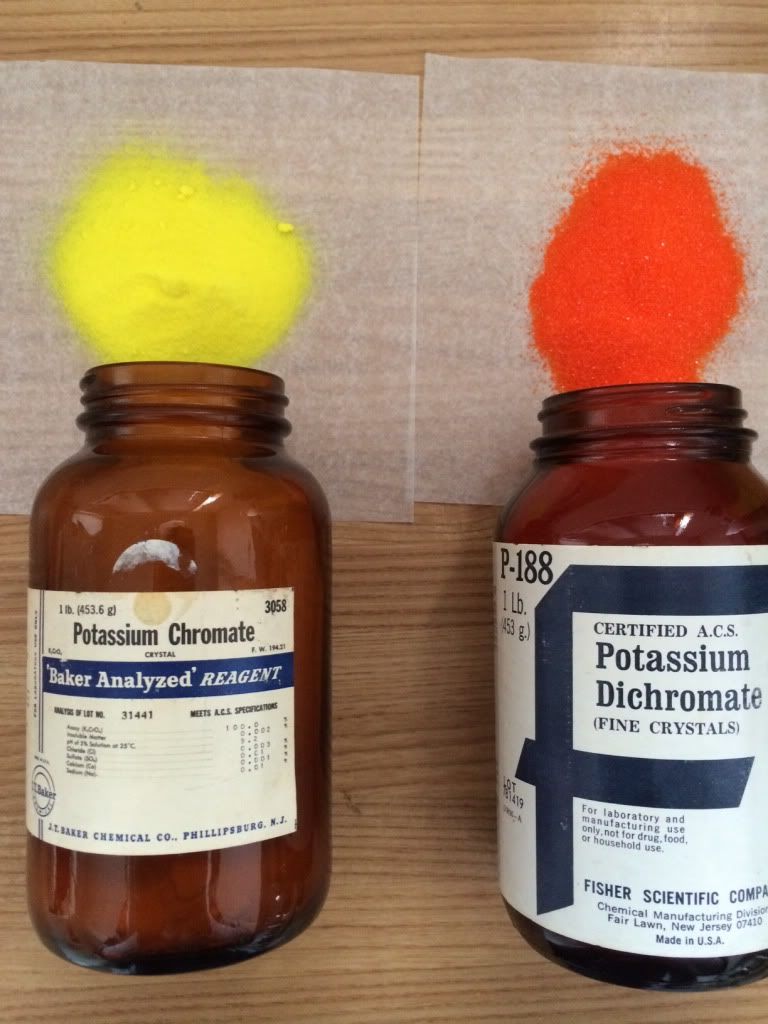
|
|
|
Chemistry_Keegan
Harmless

Posts: 45
Registered: 16-2-2013
Location: Nova Scotia
Member Is Offline
Mood: Interested
|
|
Sodium aluminate crystals that formed over a course of several weeks after all the water evaporated on it's own 

|
|
|
BromicAcid
International Hazard
    
Posts: 3246
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
So pretty, they look like artist pigments. I believe at one time some oxidizing agents were used as pigments but the medium doesn't tend to hold up
well to oxidizing agents.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I just got some potassium dichromate as well, I will post a picture of some nice big crystals as soon as I can make them, until then, it looks the
same as Mailinmypocket's.
|
|
|
NeonPulse
Hazard to Others
  
Posts: 417
Registered: 29-6-2013
Location: The other end of the internet.
Member Is Offline
Mood: Isolated from Reality! For Real this time....
|
|
Re-crystallizing some Trinitrophenol. Got a nice crop of well formed needle shaped crystals in the beaker....
 i see alot of the pics here are of some type of crystalline formations. the
silver metal on the copper wire look pretty neat. i tried the same but could not get decent close up pics. i see alot of the pics here are of some type of crystalline formations. the
silver metal on the copper wire look pretty neat. i tried the same but could not get decent close up pics.

|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by NeonPulse  | Re-crystallizing some Trinitrophenol. Got a nice crop of well formed needle shaped crystals in the beaker....
 i see alot of the pics here are of some type of crystalline formations. the
silver metal on the copper wire look pretty neat. i tried the same but could not get decent close up pics. i see alot of the pics here are of some type of crystalline formations. the
silver metal on the copper wire look pretty neat. i tried the same but could not get decent close up pics.
|
What solvent did you use? ... I prepared today few grams of TNP and then dissolved it in warm ethanol.. so now I´m waiting for crystals..
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Chromuim Chemistry
Interesting color reaction:
Control: water
#1 reaction: water with 5 drops 3%H2O2
#2 reaction: water with 5 drops 3%H2O2
+ 5 drops 30%HCl
~50 mg potassium dichromate is added to each tube.
Control is on the right, #1 reaction in the middle and #2 reaction on left.
 10 seconds after addition. 10 seconds after addition.  2 minutes after addition. 2 minutes after addition.  5 minutes after addition. 5 minutes after addition.  10 minutes after addition. 10 minutes after addition.
[EDIT] Now 15-20 minutes after the last picture was taken, #2 reaction tube is nearly colorless, a bit of unreacted potassium dichromate has settled
on the bottom, but the solution is very pale blue. The camera doesn't pick it up very well, it's slightly more colored then the picture shows.
[Edited on 31-3-2014 by Zyklonb]

|
|
|
| Pages:
1
..
7
8
9
10
11
..
77 |