| Pages:
1
..
7
8
9
10
11
..
23 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Another new study:
Impact insensitive dinitromethanide salts
J. M. Shreeve, Ling He, Damon A. Parrish and Guo-Hong Tao
Chem. Commun., 2013, Accepted Manuscript
DOI: 10.1039/C3CC46518G
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
What about the trioxane version of this ?
I have read about 135 trinitromethyl triazene, it has a very good positive oxygen balance but its unstable. So i wonder if the trioxane, although
might be less energetic, is stable.
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
This is interesting because it has high gas production and very high density. Aluminum nitride also seen to be able to be used since it has a similar
heat of formation however having a much higher density of 3.26 Can it be added in powdered form in rocket propellants ?
|
|
|
VladimirLem
Hazard to Others
  
Posts: 204
Registered: 24-5-2010
Member Is Offline
Mood: Have no fear <Vlad> is here.
|
|
Hi
I read about DiNitroGuadinine and NitroGuadinineNitrate and both can be made by the nitration of nitroguanidine (NGN needs only 60% HNO3 and DNG needs
100%+H2SO4/oleum)...but i dont want to waste such many H2SO4 on DNG and dont want to make oleum (to expensive and much much work - and fucking
dangerous btw) so i wonder if it is possible to make a too step nitration like the RDX synthesis with hexamine dinitrate...
hexamine (+HNO3)-> hexaminedinitrate (+HNO3)-> RDX
nitroguanidine (+60%HNO3)-> nitroguanidinenitrate (+HNO3+"a bit" of H2SO4)-> dinitroguanidine ??????
could that work?
other question, there is not much information about NGN...only some translation that says it would be more powerful than DNG or
ammoniumdinitroguanidine...can this be? if yes, <nevermind>@first question 
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
NGN
NGN prepare a like RDX?
The answer will learn the following: Check it out. Try it. It's the fastest way. Fastest response. A completely accurate. A write how it turned out.
LL
|
|
|
ecos
Hazard to Others
  
Posts: 464
Registered: 6-3-2014
Member Is Offline
Mood: Learning !
|
|
did anyone succeed in synthesis any of these new EM ? I went through some papers posted here and found them very complex.
if yes , please share your experience.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
4,4,8,8-Tetranitroadamantane-2,6-diyl Dinitrate: A High-Density Energetic Material
Yifei Ling, Xiaoli Ren, Weipeng Lai and Jun Luo
European Journal of Organic Chemistry, 2015; An ASAP article first published online (22. Jan. 2015)
DOI: 10.1002/ejoc.201403449
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Nicodem  | 4,4,8,8-Tetranitroadamantane-2,6-diyl Dinitrate: A High-Density Energetic Material
Yifei Ling, Xiaoli Ren, Weipeng Lai and Jun Luo
European Journal of Organic Chemistry, 2015; An ASAP article first published online (22. Jan. 2015)
DOI: 10.1002/ejoc.201403449 |
Thanks Nicodem,
Just for those curious about it:
Quoted from the link:
Abstract:
The novel high-performance energetic material, 4,8,8-tetranitroadamantane-2,6-diyl dinitrate has been synthesized and fully characterized. The
synthetic strategy features the construction of the adamantane skeleton with different functional groups at adjacent methylene carbon atoms to
overcome the problems associated with the ortho effect and steric crowding, and is amenable to the synthesis of other polynitroadamantanes. The
experimentally determined physical properties as well as the calculated detonation properties indicate that this compound exhibits high thermal
stability (220 °C), high density (1.852 g /cm³), and excellent detonation velocities (8529 m /s) and pressures (33.43 GPa).
And for those who likes it with images:
4,4,8,8-Tetranitroadamantane-2,6-diyl Dinitrate
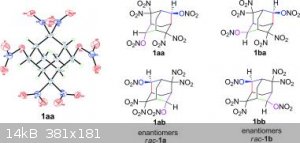
I think the 2,2,4,4,6,6,8,8-octanitroadamantane would be also interesting and there are two methylene left free that would render possible the making
of a tetranitroadamantane-tetrayl-tetranitrate or a dodecanitroadamantane with each methylene holding two nitro groups...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by Nicodem  | 4,4,8,8-Tetranitroadamantane-2,6-diyl Dinitrate: A High-Density Energetic Material
Yifei Ling, Xiaoli Ren, Weipeng Lai and Jun Luo
European Journal of Organic Chemistry, 2015; An ASAP article first published online (22. Jan. 2015)
DOI: 10.1002/ejoc.201403449 |
Thanks Nicodem,
Just for those curious about it:
Quoted from the link:
Abstract:
The novel high-performance energetic material, 4,4,8,8-tetranitroadamantane-2,6-diyl dinitrate has been synthesized and fully characterized. The
synthetic strategy features the construction of the adamantane skeleton with different functional groups at adjacent methylene carbon atoms to
overcome the problems associated with the ortho effect and steric crowding, and is amenable to the synthesis of other polynitroadamantanes. The
experimentally determined physical properties as well as the calculated detonation properties indicate that this compound exhibits high thermal
stability (220 °C), high density (1.852 g /cm³), and excellent detonation velocities (8529 m /s) and pressures (33.43 GPa).
And for those who likes it with images:
4,4,8,8-Tetranitroadamantane-2,6-diyl Dinitrate
I think the 2,2,4,4,6,6,8,8-octanitroadamantane would be also interesting and there are two methylene left free that would render possible the making
of a tetranitroadamantane-tetrayl-tetranitrate or a dodecanitroadamantane with each methylene holding two nitro groups... |
Article is to be found here
4488-tetranitroadamantane-26-diyl dinitrate
Thanks to Mayko via request in References/ Wanted References 
[Edited on 20-3-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
A few early view Angewangte articles:
Sulfates-Based Nanothermites: An Expanding Horizon for Metastable Interstitial Composites
Comet, M., Vidick, G., Schnell, F., Suma, Y., Baps, B. and Spitzer, D.
Angew. Chem. Int. Ed., (2015) 54: 4458–4462.
doi: 10.1002/anie.201410634
The Many Faces of FOX-7: A Precursor to High-Performance Energetic Materials
Gao, H. and Shreeve, J. M.
Angew. Chem. Int. Ed. (2015).
doi: 10.1002/anie.201501973
Energetic Materials with Promising Properties: Synthesis and Characterization of 4,4′-Bis(5-nitro-1,2,3-2H-triazole) Derivatives
He, C. and Shreeve, J. M.
Angew. Chem. Int. Ed. (2015).
doi: 10.1002/anie.201412303
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Thanks Nicodem!
The abstract on sulfate based nano thermite article mentions burn rates in a range, high end is given at 2,500 meters/second?! That's faster than
several ammonium nitrate based high explosives in common use- Whee! And water of crystalization is considered a FEATURE by author, not a bug?
Time to re-consider sulfate oxidizers, The WiZ may not have covered all possibilities...
What's possible to achieve as an amateur using OTC materials for a reasonably low cost, easily cast/worked DENSE refractory material. I have a little
modification of the pyronol torch/linear thermite steel cutting charge family of devices in mind.
(edit)
How to convince micro scale particles of Aluminum to behave like nano scale?
http://www.dtic.mil/get-tr-doc/pdf?AD=ADA534332
[Edited on 3-4-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
Bert, the 2500 m/s nano thermite mentioned in Sulfates-Based Nanothermites is probably a CuO/Al mix:
| Quote: | While in the literature CuO/Al thermites have not been prepared by reactive
milling or sol-gel methods, unique to CuO/Al thermite is a new synthesis
methodology by Shubhra Gangopadhyay called self-assembly [17]. In this
method, the authors coated copper nanorods (20x100 nm) with a coordinating
polymer (P4VP) followed by coating the coated rods with 80 nm aluminum
powder. The result of this self-assembly process is the thermite with the highest
combustion velocity known of 2400 m/s. |
Nanoscale Aluminum - Metal Oxide (Thermite) Reactions for Application in Energetic Materials
Piercey, D. G. and Klapötke, T. M.
http://www.wydawnictwa.ipo.waw.pl/cejem/2-2010/full/klapotke...
The water of crystalization was a problem when I made CuSO4/Mg flash powder. The hydrated mixture surely burns, but the anhydrous mixture
explodes with immense violence when heated. The water lowers the ignition point and increases energy content of the mixture though, so why not. If it
wasn't for the slow oxidation of magnesium in water, this mixture could have some use:
https://youtu.be/ix-OTDv0i8s?t=1m8s
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
hi community
somebody mentioned the Energetic Nitrate Ester : / 2,3-Bis-hydroxymethyl-2,3-dinitro-1,4-butanediol tetranitrate / the synthesis isn't that
difficult if you have / 2,2-Dimethyl-5-nitro-1,3-dioxan-5-yl)methanol / needed for the first synthesis.
I found a chinese PDF about this explosiv Attachment: phpJ8WtHo (540kB)
This file has been downloaded 2383 times
started from Nitromethan 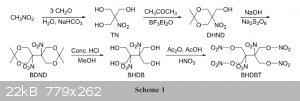
Maybe someone is able to write a synthesis???
Star from 2,2-Dimethyl-5-nitro-1,3-dioxan-5-yl)methanol
Attachment: Synthesis of an Energetic Nitrate Ester.pdf (2.5MB)
This file has been downloaded 1176 times
[Edited on 10-4-2015 by Mr.Greeenix]
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Synthesis of 2,2-Dimethyl-5-nitro-1,3-dioxan-5-yl)methanol is very easy if you have nitromethane and 2,2-dimethoxypropane (the dimethyl acetal of
acetone) along with some (para)formaldehyde and a few common reagents. There are patents for making the 2,2-dimethoxypropane from acetone and the
alcohol, but I never got it to work beyond trace amounts (enough to smell, but nothing more).
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The synthesis is even easier from 1.2-dinitroethane and formaldehyde...
O2N-CH2-CH2-NO2 + 4 CH2=O -base-> (HOCH2)2C(NO2)-C(NO2)(CH2OH)2
Based on the same principle as nitroisobutyl-triol from nitromethane and formaldehyde...
CH3-NO2 + 3 CH2=O -base-> (HOCH2)3C-NO2
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
But then, where to get the DNE? I haven't seen this for sale anywhere, and IIRC it is unstable in storage. I haven't come across a reasonable
synthesis either.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
from ethylene dibromide:
Br-CH2-CH2-Br + AgNO2 --> O2N-CH2-CH2-NO2 (36%) + ONO-CH2-CH2-ONO (16%) + ONO-CH2-CH2-NO2 (48%)
from tartric acid fumaric acid or maleic acid:
HO2C-CHOH-CHOH-CO2H -halogenation-> HO2C-CHX-CHX-CO2H
HO2C-CH=CH-CO2H (cis or trans) + X2 -->HO2C-CHX-CHX-CO2H
HO2C-CHX-CHX-CO2H + NaNO2 + urea -DMF-or-DMSO->HO2C-CH(NO2)-CH(NO2)-CO2H + HO2C-CH(ONO)-CH(NO2)-CO2H + HO2C-CH(ONO)-CH(ONO)-CO2H
alfa nitro carboxylic acid performs spontaneous decarboxylation...
HO2C-CH(NO2)-CH(NO2)-CO2H --> O2N-CH2-CH2-NO2 + 2 CO2(g)
HO2C-CH(ONO)-CH(NO2)-CO2H --> O2N-CH2-CH(ONO)-CO2H + CO2(g)
in mild acidic media, only the dinitro compound will be unsoluble
Urea increases solubility of nitrite and avoid side nitrosation reaction of the nitrocompound otherwise you would get aswel nitrolic acids...
R-CH2-NO2 + R'-ONO --> R-CH(-N=O)(NO2) + R'OH
R-CH(-N=O)(NO2) <--==> R-C(NO2)(=N-OH)
[Edited on 11-4-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
With those extra steps, I think that the litterature path is about as good, but it would certainly be an interesting experiment.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
There is also an electrolytic dimerization of secondary nitrocompounds ammonium salts... 
2 (R)2C(NO2)NH4 -electrolysis-> (R)2C(NO2)-C(NO2)(R)2
Based on that principle:
CH3-NO2 + 2 CH2=O --> (HOCH2)CH-NO2
(HOCH2)CH-NO2 + NH3 --> (HOCH2)C=N(O)-ONH4
2 (HOCH2)C=N(O)-ONH4 -electrolysis-> (HOCH2)C(NO2)-C(NO2)(CH2OH)2
Maybe the alcool function has to be protected first against electro-oxydation by acetylation...the resulting dinitro-tetraacetyl ester would then be
unsoluble while the ammonium salt would be soluble, yielding and easier isolation.
[Edited on 12-4-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
I put the detailed syn. of / 2,2-Dimethyl-5-nitro-1,3-dioxan-5-yl)methanol / from the chines paper in a translater.
That's my result.
(sorry for the realllly bad English) Maybe someone can translate it into good English
methyl nitro-methane (TN):
In the bottom flask fitted to 37% of the formaldehyde in water (327.5 G, 4
mol), and water bath heated to 50 degrees C, add the appropriate amount baking soda to adjust
pH 8.0 sites, increase the temperature to 55 degrees C, drop the nitro-methane (76.9 G, 1.25
mol), and maintain pH sites 8.5, the contribution that the agitation response 40 min to drop to a 10%
of the sulfuric acid adjustment response fluid pH sites 5, heating the reaction fluid temperature up to
80 degrees C, reducing enrichment, cooling, and by a large white needle-like crystals, the
Filter, vacuum drying, and in 3 of methyl nitro-methane 160.8 G / yield 85.2%. / m.p. 152~153 Celsius / C4H9NO5
2,2-Dimethyl-5-nitro-1,3-dioxan-5-yl)methanol:
Adds three methylol nitromethane in the round flask (120.8 g, 0.8 mol), acetone (176.4 mL), stirring,
heating in water bath to 60 degrees Celsius. After three hydroxy A base nitromethane dissolve
completely, ices the water bath to make the solution cool to 10 degrees Celsius by below, the
separation massive acicular crystals. Adds by drops the boron trifluoride etherate solution
(189.8 g, 0.84 mol), Canada finishes, responded 5 min the heating in water bath to
50~55 degrees Celsius, will respond that the fluid poured into the sodium hydrogencarbonate in the
ice water saturated solution, stirring, regulator solution pH close neutrality, pulled out filters
the white solid, the ice water washed 2 times, after the vacuum drying,
2,2- dimethyl - 5- methylol - 5- nitro groups - 1,3- two oxygen mixed hexamethylene 133.1 g,
yield 87.1%. m.p. 130~131 degrees Celsius; C7H13NO5
The only problem is "Boron trifluoride diethyl etherate" but I think some are able to buy it.
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
If you use 2,2-dimethoxypropane instead of acetone as I mentioned above, there is no need for BF3. Of course, 2,2-DMP isn't exactly OTC either...
Anyway, the article can be had in english and is also discussed elsewhere on this site, so you don't have to go through a machine translation.
Edit: Now I'm confused: You posted the article in english yourself just a few posts back, so what is it you are asking?
[Edited on 22-4-2015 by Microtek]
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
I posted 2 articels.
1 is in English and starts from 2,2-Dimethyl-5-nitro-1,3-dioxan-5-yl)methanol
and the 2 one is the chinese paper were one synthesis route ist improved from 85->95% and it starts from Nitromethan.
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
Oh, OK. Tris-nitro (tris(hydroxymethyl)nitromethane) is easy to make in high yield. It is used as a biocide, and there are lots of patents on its
preparation.
The second step, I found to be not so easy. In the end I just ordered the 2,2-DMP through Sigma-Aldrich after having tried (unsuccesfully) to
synthesize it myself. There are patents about that part also, but in my experience they didn't work as advertized.
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
It was mentioned before but here is the pdf of Tetranitro-propane diurea and
an other very insensitiv compound
Attachment: TNPDU.pdf (110kB)
This file has been downloaded 1357 times
[Edited on 25-4-2015 by Mr.Greeenix]
|
|
|
Mr.Greeenix
Harmless

Posts: 40
Registered: 8-4-2015
Member Is Offline
Mood: No Mood
|
|
Hydrazinium 5-Aminotetrazolate
* incredible high value (9516 m/s) was obtained for the detonation velocity
* it is incredibly insensitiv
* easy preparation
Attachment: Hydrazinium 5-Aminotetrazolate.pdf (498kB)
This file has been downloaded 1154 times
What do you think?
[Edited on 20-5-2015 by Mr.Greeenix]
|
|
|
| Pages:
1
..
7
8
9
10
11
..
23 |