| Pages:
1
..
7
8
9 |
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by MagicJigPipe
So, what about a tanning bed light since they are specifically designed to emit light in those wavelengths? And you wouldn't have to modify the bulb.
|
Mercury vapour light - 175W, arc about 5 cm in length, cost for fixture and lamp about $40 US.
Tanning bed lamp - 100 W, 175 cm in length, cost $14 US, needs fixture.
It's going to be easy to get most of the light from that no more than finger length arc onto the apparatus. But a 175 cm long tube ... can you draw
me a picture of your setup?
You want the clear mercury vapour lamps, not the white phosphor coated ones.
As for the reflector, consider covering on side of a sheet of light cardboard or artboard with aluminium foil. It doesn't need to be an image quality
mirror.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
Oh, I have some shorter (~2ft) tanning bulbs with the special fixture they came in. I got them from an old sit-down tanning bed in a gym I used to
work at. I suppose because of the size issues that the mercury vapor lamp would be better. I just am still uneasy about 100w being able to work
well. I won't know 'til I try. I'll do a small scale setup with the equipment I already have and if it's too cumbersome then I'll opt for the Hg
vapor.
*Edit*
Sorry for the "archaic" measurements. 2ft = 60.96cm
[Edited on 12-12-2007 by MagicJigPipe]
[Edited on 12-12-2007 by MagicJigPipe]
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Reno.456
Harmless

Posts: 1
Registered: 24-12-2007
Member Is Offline
Mood: Dangerous!!!
|
|
Chlorine
I may have miss the point but you can heat up salt (NaCl) then run a current through it:
2 NaCl---->2 Na + Cl2
So just collect the chlorine and chuck the Sodium in someone elses pond!
|
|
|
chloric1
International Hazard
    
Posts: 1142
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
LOL!
Good luck to you Reno! 
First you need a hellafied gas burner to heat the sodium chloride to 801 Celsius so it will melt. Next you will have a nice engineering feat of
trying to keep the very hot reactive chlorine gas and molten sodium from exploding in your face 
Fellow molecular manipulator
|
|
|
Klute
International Hazard
    
Posts: 1378
Registered: 18-10-2006
Location: France
Member Is Offline
Mood: No Mood
|
|
Adding CaCl2 will diminish the mp of the eutetic mixture IIRC, and Ca metal cannot be produced with iron electrodes. Such an electrolysis should be
conducted with a flow of inert atmospher to protect the melted Na, which would seperate as a top layer, while it is transfered out of the cell and
cooled away from atmospher. This is done in the industry, and apparently gives Na of good purity. Adapting this to a home lab is completly another
story though. I guess it could be done by investing money and time. There should be some fairly detailed patents around.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
OTC pool chorinating compounds and data
http://www.rhtubs.com/chlorine.htm
.
|
|
|
a1dirkscience
Harmless

Posts: 4
Registered: 11-12-2008
Member Is Offline
Mood: frustrated
|
|
Back to the topic of generating cl2 gas on a long term basis and having it clean and dry.
This is how i built my generator. a five gallon bucket, 4 anodes, 2 cathodes, equal in total mass. The electrodes are carbon/graphite. 4 small water
drinking bottles, some 1/4" drip tubing and connectors. some liquid plastic to seal everything up. hot glue duct tape and zip ties to put it all
together.
so 4 cl2 collectors and the hyrodgen was left to float away. filled the bucket with distilled H2O and NaCl. Ran the cl2 line in to H2so4.
power supply is a 12 volt dc battery charger set on the start cycle.
after about an hour the cl2 gas comes up to pressure and flows like crazy !!!!!!
as long as there is more Cl than O it puts out cl2 gas , just add a little more NaCl now and then.
Ran it for 15 hours and is still blowing green gas.
heres my question , how can I remove any Oxygen that might be in the gas ? Some on in the thread said it was 0.01 % .
In my books they say that O will only start coming out after the Cl is below the O ???
Im trying to make chloral for Melzers reagent.
And I did make some but no where near enough.
I'm thinking that the Oxygen stoped the chains from forming ?
|
|
|
a1dirkscience
Harmless

Posts: 4
Registered: 11-12-2008
Member Is Offline
Mood: frustrated
|
|
P.S. you can get the electrodes at any welding store . as for carbon arc cutting rods. The copper plating peals off very easy and then you have 12"
electrodes any diameter you want
|
|
|
slinky
Harmless

Posts: 39
Registered: 14-9-2010
Member Is Offline
Mood: No Mood
|
|
Chlorine free radical halogenation light source
Why not use a blacklight? It gives off the appropriate wavelength of light "320 and 400 nanometres with a peak at 365 nanometres" The party lights
are supposed to block all of the sunburn causing UVB and UVC light. It also blocks all irrelevant light between 400-700 nm." Only UVA is given off. It
doesn't get hot to the touch either.
I was able to purchase one for 10 USD with a ballast. It's 60.96 cm ( 24 inches ) long. It's light weight and I can easily clamp it vertically to a
stand. This will make it easy to expose refluxing liquids mixed with chlorine gas to UVA light. As an added benefit all of the refluxing liquid and
chlorine gas should glow like a glow stick 
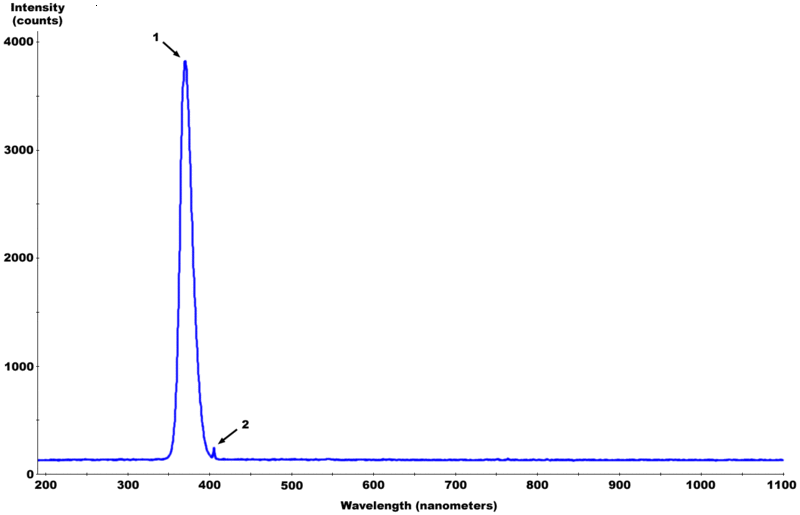
^ Fluorescent Black Light spectrum with peaks labelled
https://secure.wikimedia.org/wikipedia/en/wiki/Black_light
"Black light fluorescent tubes are typically made in the same way as normal fluorescent lights except that only one phosphor is used and the normally
clear glass envelope of the bulb may be replaced by a deep-bluish-purple glass called Wood's glass. Wood's glass is a nickel-oxide–doped glass,
which blocks out almost all of the visible light, that is, energy in the electromagnetic spectrum with a wavelength of between about 400 and 700
nanometers."
https://secure.wikimedia.org/wikipedia/en/wiki/Wood%27s_glas...
"Wood's glass is special barium-sodium-silicate glass incorporating about 9% nickel oxide. It is a very deep violet-blue glass, opaque to all visible
light rays except longest red and shortest violet. It is quite transparent in the violet/ultraviolet in a band between 320 and 400 nanometres with a
peak at 365 nanometres, and a fairly broad range of infrared and the longest, least visible red wavelengths."
http://science.howstuffworks.com/innovation/black-light1.htm
"A tube black light is a basically a fluorescent lamp with a different sort of phosphor coating. This coating absorbs harmful shortwave UV-B and UV-C
light and emits UV-A light (in the same basic way the phosphor in a fluorescent lamp absorbs UV light and emits visible light). The "black" glass tube
itself blocks most visible light, so in the end only benign long-wave UV-A light, along with some blue and violet visible light, passes through. "
http://www.differencebetween.net/technology/different-betwee...

http://www.skincancer.org/understanding-uva-and-uvb.html

[Edited on 11.23.2010 by slinky]
[Edited on 11.23.2010 by slinky]
|
|
|
slinky
Harmless

Posts: 39
Registered: 14-9-2010
Member Is Offline
Mood: No Mood
|
|
I attempted free radical chlorination of toluene as per lien1's post here:
https://www.sciencemadness.org/whisper/viewthread.php?tid=10...
Instead of using a mercury vapour lamp I used a blacklight. As you can see from my previous post, I was quite confident that the blacklight would work
because although the light is less intense it is indeed within the specified light spectrum. Post chloronation a fractional distillation was commenced
with an insulated snyder column at atmospheric pressure. After the toluene finished coming over the thermometer climbed up to about 163 and there it
sat until the temperature climbed to about 190. At around 190 some high boilers started condensing which were a blue/violet colour. My experiment
failed to produce products which are consistent with free radical chloronation conditions. Per Organic Chemisrty 2nd edition by Morrison and Boyd p.
386 the products synthesised are consistent with chloronation in the dark.




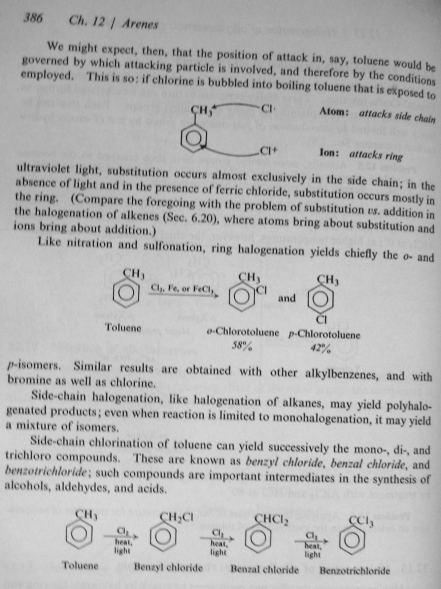
[Edited on 1.13.2011 by slinky]
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
That sucks, what a waste of time for you, i think the light intensity from black light is fairly pathetic, also borosilicate will absorb around half
your UV leaving you with not much happening. Buy a merc vapour globe and ballast, 250-400 watt is good. If it has a white phospour as most do you need
to simply remove the outer glass shell with a careful scratch and tap then run the bulb with the outer shell. removed, it generates lots of ozone and
will sun burn you within minures so have yourself protected from this. Get the globe as close as possible to your condenser, remembering this reaction
occurs in the vapour phase. If you do not notice the bp going up significantly during the process something is wrong
|
|
|
slinky
Harmless

Posts: 39
Registered: 14-9-2010
Member Is Offline
Mood: No Mood
|
|
Thanks panache, I have a large ballast on the way. It should be arriving within a few days. I'd have to agree, the intensity of the blacklight was
very weak compaired to a mercury vapour bulb. I thought that allthough less light was given off the fact that all of the light given off was within
the desired spectrum would be make the blacklight sufficently powerful and a safer higher efficency route. I was incorrect.
Thanks for the tips regarding the bulb. I'll likely use a transparent bulb. I'm thinking about cutting a hole in an extra large stainless steel mixing
bowl so I can mount the bulb to the center of it. The mixing bowl will serve a dual role acting as a light shield for me and acting in the same manner
as a parabolic dish. By adjusing the location of the bowl I will be able to concentrate the bulk of the light/heat energy to a small focused area
within the refluxing toluene.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Don't know if it's been mentioned, expect it has, but there are chlorine generators available that run on salt water and electricity.
A membrane separating the electrodes prevents the chlorine from recombining with the electrolyte.
This member is either a.) asbestos b.) perflurosulfonic acid polymer (PFSA).
Given the issues with asbestos, might be best to avoid that. If someone could find a surplus sheet of PFSA, maybe we could chop it up and split it
between those interested.
You may even be able to buy it from the guys who produce chlorine generators for pools, as a replacement part.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Bubble it through 10% NaOH, this is what's done industrially and it doesn't involve it getting airborne and then recaptured.
You may have more luck with the CCl3 / CCl4 if the reaction is warmed - perhaps in a vapour phase - as it takes more and more effort to add the
chlorines as you go up (although I seem to remember, perhaps incorrectly, that the final chlorine is easier than the third).
We did some theory about this precise series in A-Level chemistry, and the teacher mentioned that he used to knock up some chloroform when kids
brought injured pigeons in from the playground, to humanely euthanise them if they were in a bad way.
Take care using free radical methods, the results can occur at an eye popping rate when the light goes on. 
[Edited on 13-1-2011 by peach]
|
|
|
Arthur Dent
National Hazard
   
Posts: 553
Registered: 22-10-2010
Member Is Offline
Mood: entropic
|
|
Quote: Originally posted by slinky  | | I'm thinking about cutting a hole in an extra large stainless steel mixing bowl so I can mount the bulb to the center of it.(...)
|
I would suggest that you look into a reflector made of aluminium. I've noticed that most if not all UV reflectors i've seen are made of aluminium.
Perhaps stainless steel, although highly reflective, might absorb some of the UV spectrum(?).
An aluminium bowl/cone/reflector would probably be lighter and easier to work, cut and secure to a frame anyway. 
Robert
--- Art is making something out of nothing and selling it. - Frank Zappa ---
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
They may be using aluminium due to the significantly lower cost of buying, pressing and folding it. Damn cheap skates! 
Aluminium reflectors around hot lights tend to end up dull with oxide I've found.
Whilst fixing the bathroom up over christmas, I managed to break THREE lights trying to work with the light ring off.
Should have got myself one of those 400W Halide Wobblelights, those things look absolutely perfect!
[Edited on 13-1-2011 by peach]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Hg vapour lights
Sorry if this is not new, however, I am not going to read trough
pages of info.
I am not sure this will still work as I believe they may have changed
the design to stop this.
With the older Hg vapour lights you could remove the outer glass
bulb exposing the quartz tube - allowing shorter wave lengths
that were absorbed by the glass bulb out. [Remember not to touch
the quartz with your bare fingers!]
Or you could buy one of those old Hg vapour tanning lights
on eBay. Please don't fry la eyeballs. Love the smell of the
ozone they produce. Or as me coworker Screaming Carl used to
say years ago. I can smell the ozone. - I was using the Zerox
machine.
Say anyone remember the old wet copiers? You had to hand
the copies out to dry. And the Termolfax copiers? They used heat and made slimy copies.
http://en.wikipedia.org/wiki/Thermofax
"The Thermofax process was temperamental. The coated paper
tended to curl, and being heat-sensitive, copies were not archival.
[5] The darkness setting was tricky to adjust, and drifted as the
machine warmed up. Copy darkness often varied across a page,
some portions of the text being too light and others too dark. Since
the heat absorption of ink does not necessarily correlate with its
visible appearance, there were occasional idiosyncrasies; some
inks that looked nearly black to the eye might not copy at all, and
an exposure setting that worked well for some originals might
require a change to make usable copies with another."
Amen.
djh
----
Don't worry about loosing
you mind. When you do you
will never notice.
Screaming Carl
|
|
|
slinky
Harmless

Posts: 39
Registered: 14-9-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by peach  |
Bubble it through 10% NaOH, this is what's done industrially and it doesn't involve it getting airborne and then recaptured.
[Edited on 13-1-2011 by peach] |
Working with chlorine outside of a hood is fine so long as you respect the chlorine and HCl and capture it correctly.
When I first started chloronating the toluene I had a thin glass tube bubbling the offgassing HCl through a 10% NaOH solution. A lot of the HCl was
not reacting with the NaOH and escaping with this method. I remembered that Lambda-Eyde used an inverted glass funnel to capture bromine fumes in the
bromine thread.
https://www.sciencemadness.org/whisper/viewthread.php?tid=69...

I promptly switched to the inverted glass funnel and placed a heavy flask weight around the neck to keep the funnel submerged in a deep NaOH solution.
This worked great. The sunken weighted funnel causes the HCl to have to push down hard on the surface of the NaOH in an effort to escape. I adjusted
the chlorine generator drip rate (about 2 drops per second ) to the rate that the solution could accept the offgassing HCl. There is no bubbling at
all in the scrubber once the atmosphere within the glassware becomes saturated with HCl. This works much better than a tube bubbling imo. I just
checked the PH from time to time and added more NaOH as needed.
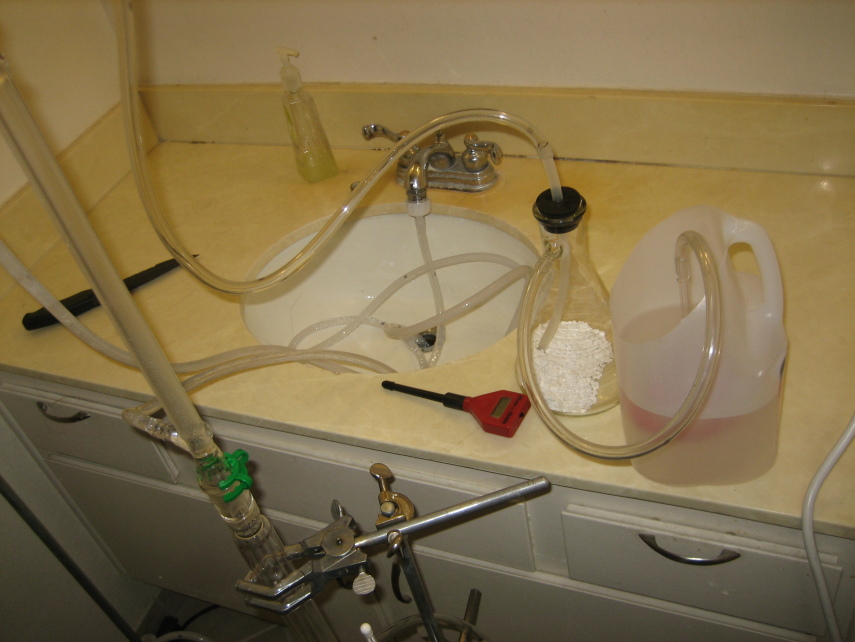

[Edited on 1.14.2011 by slinky]
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
Yep, you need to bubble at a rate that is applicable to the scrubber - in the same way that distilling something as fast as possible is likely not
doing the results any favours.
If you want to get really fancy, stick some indicating solution in the scrubber for a colourful, visual feedback on what's happening.
It's not only important in terms of capturing the gas at the outlet, getting gases to dissolve or react with what you're primarily trying to do can be
difficult because they need to absorb. For example, you're trying to make hydrochloric acid by bubbling HCl. Two people do the same thing with the
same amounts of material, one runs it as slow as possible, the other doesn't care. In the latter example, they're probably going to end up loosing a
fair amount of it as it bubbles out before it gets a chance to fully dissolve.
[Edited on 14-1-2011 by peach]
|
|
|
slinky
Harmless

Posts: 39
Registered: 14-9-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Arthur Dent  |
I would suggest that you look into a reflector made of aluminium. I've noticed that most if not all UV reflectors i've seen are made of aluminium.
Perhaps stainless steel, although highly reflective, might absorb some of the UV spectrum(?).
|
I had the same opinion as peach. That manufacturers were just cheap skates. They don't make 'em like they used to. I was also concerned about the
aluminium corroding. Well I did some digging around on some aquarium forums and it turns out that I was again wrong.
----------------------------------------------------------------------------------------
Specular Reflection
At the other extreme is mirror or specular reflection exhibited by shiny metal surfaces such as chrome, silver or pure aluminium.
It is most important to realise that although specular reflections produce a clear image in the surface of the material, the actual amount of light
reflected may be deceptively low.
A matt white painted surface, for instance, has a reflectance of 85% to 90% compared with only 60% specular reflectance from a polished stainless
steel surface, while polished aluminium will be approximately 85%.
----------------------------------------------------------------------------------------
Source: http://www.thornlighting.co.uk/gb/en/res_radiance_reflection...
Aluminium is indeed a superior reflector to stainless steel. These aquarium guys take reflectors seriously because their lights are running so often
that they can't afford to lose a few % of reflectance.
Here's a breakdown of what surface textures and finishes reflect well or not and their prices. http://anomet.com/cgi-bin/online/storepro.php
In the end I decided to go with a matt white painted surface. It should protect the metal from corrosion the best and can be reapplied. If were to use
aluminium it would likely corrode and destroy the high polish eliminating the reflector's efficiency. Matt white's performance is comparable to
aluminium and is much better than stainless steel.
|
|
|
| Pages:
1
..
7
8
9 |