| Pages:
1
..
81
82
83
84
85
..
104 |
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
I seem to think that most ordinary titrations typically involve ~0.1N solutions.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Is it really that hard to get Hg? I can buy mercury thermometers at Walgreens. They have a mercury warning and a clearly-visible bulb of silvery
liquid so I assume they must contain it.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I suspect that cinnabar might be a variety of tumeric powder in actuality.
In the description it says it's curcuma, and that you can put it under a kids pillow to make him sleep.
Might be worth a try, but I am skeptical.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
I have googled this question with no luck. Does carbon dioxide displace aluminate?
Meaning will carbon dioxide decompose a concentrated solution of sodium aluminate to produce sodium carbonate/bicarbonate and aluminium hydroxide?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by wg48  | I have googled this question with no luck. Does carbon dioxide displace aluminate?
Meaning will carbon dioxide decompose a concentrated solution of sodium aluminate to produce sodium carbonate/bicarbonate and aluminium hydroxide?
|
Yes. The Ksp for aluminum hydroxide and the Kf are practically reciprocals of each other, so the eq'm constant for the reaction of the hydroxide ion
with aluminum hydroxide to give the complex ion is close to one. At eq'm, the concentration of the complex ion will be the same as unreacted
hydroxide ion (so if you saturate 1 M NaOH with aluminum hydroxide, it will be about 0.5 M sodium hydroxide and 0.5 M sodium aluminate, with a pH of
13.7ish). Carbon dioxide will convert the hydroxide ion into carbonate and then to bicarbonate, lowering the pH. if it lowers the pH to 10 (close to
the pH of a bicarbonate/carbonate buffer), the hydroxide concentration will be 0.0001 M, and so will the aluminate concentration.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by wg48  | I have googled this question with no luck. Does carbon dioxide displace aluminate?
Meaning will carbon dioxide decompose a concentrated solution of sodium aluminate to produce sodium carbonate/bicarbonate and aluminium hydroxide?
|
Yes. The Ksp for aluminum hydroxide and the Kf are practically reciprocals of each other, so the eq'm constant for the reaction of the hydroxide ion
with aluminum hydroxide to give the complex ion is close to one. At eq'm, the concentration of the complex ion will be the same as unreacted
hydroxide ion (so if you saturate 1 M NaOH with aluminum hydroxide, it will be about 0.5 M sodium hydroxide and 0.5 M sodium aluminate, with a pH of
13.7ish). Carbon dioxide will convert the hydroxide ion into carbonate and then to bicarbonate, lowering the pH. if it lowers the pH to 10 (close to
the pH of a bicarbonate/carbonate buffer), the hydroxide concentration will be 0.0001 M, and so will the aluminate concentration.
|
Thanks for the reply.
What is the Kf constant? I assume it not the freezing point depression constant.
So is this correct: if I attempt to dissolve NaAlO4 in water, half of the Al will be deposited as the hydroxide? Does that also mean that if I
attempt to dissolve excess Al in a NaOH solution it will eventually deposit Al(OH)3?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Kf = formation constant, i.e. the equilibrium Al(OH)3 + OH- >><< Al(OH)4- or somesuch. Actually I'm not sure what the formation reaction
is defined as in this case.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Kf is the eq'm constant for Al(3+) + 4 OH(-) = Al(OH)4(-)
The reaction Al(OH)3 + OH(-) = Al(OH)4(-) will have an eq'm constant of about 1, so the concentrations of hydroxide and the complex ion will be equal
at equilibrium (assuming you've added enough aluminum hydroxide to saturate the solution). If you dissolve pure NaAl(OH)4 in water, you will need to
add an equal amount of NaOH to prevent the precipitation of Al(OH)3.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Σldritch
Hazard to Others
  
Posts: 309
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Does anyone have solubility data for Sulfamic acid and/or various Sulfamates?
Edit: Thanks a lot Walruslover
[Edited on 13-3-2018 by Σldritch]
|
|
|
walruslover69
Hazard to Others
  
Posts: 231
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
Sulfamic acid
12.8 wt% in water at 0 deg C; 17.57 wt% in water at 20 deg C; 22.77 wt% in water at 40 deg C; 0.1667 wt% in formamide at 25 deg C; 0.0412 wt% in
methanol at 25 deg C; 0.0167 wt% in ethanol (2% benzene) at 25 deg C; 0.0040 wt% in acetone at 25 deg C; 0.0001 wt% in ether at 25 deg C
Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V21 950
[Edited on 13-3-2018 by walruslover69]
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Question
I know that highly alkaline solutions damage glass frits from vacuum filtration systems. What is the maximum pH a solution can have without noticeably
damaging the frit?
[Edited on 15-3-2018 by CobaltChloride]
|
|
|
RogueRose
International Hazard
    
Posts: 1592
Registered: 16-6-2014
Member Is Offline
|
|
Lead acetate turns cloudy when added to water - but not when making lead acetate
So when I've made lead acetate I get a crystal clear solution and when I reduce it it stays clear even at super saturation point - it may get a slight
bit yellowish, only the slightest but I would consider it clear.
If I add some of the dried lead acetate to water I get a cloudy/milk solution. When drying the compound then I remove all of it from the evaporation
dish, then add water to clean or get the rest of the remaining compound off the dish, it is milky white - not opaque but cloudy white.
I've thought maybe this is lead carbonate but if that is the case, would lead acetate turn to lead carbonate if it were left to sit out? Where does
the acetate go if it is replaced with CO2? This doesn't seem like a balanced equation and IDK what is happening here.
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Answer
Quote: Originally posted by RogueRose  | So when I've made lead acetate I get a crystal clear solution and when I reduce it it stays clear even at super saturation point - it may get a slight
bit yellowish, only the slightest but I would consider it clear.
If I add some of the dried lead acetate to water I get a cloudy/milk solution. When drying the compound then I remove all of it from the evaporation
dish, then add water to clean or get the rest of the remaining compound off the dish, it is milky white - not opaque but cloudy white.
I've thought maybe this is lead carbonate but if that is the case, would lead acetate turn to lead carbonate if it were left to sit out? Where does
the acetate go if it is replaced with CO2? This doesn't seem like a balanced equation and IDK what is happening here. |
It may be that your water has some chloride or sulfate and forms insoluble salts with Pb II. I know that some brands of distilled water have enough
chloride to make a cloudy solution when mixed with silver nitrate (from a NileRed video on the silver mirror).
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Everything that forms an insoluble carbonate does this?
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
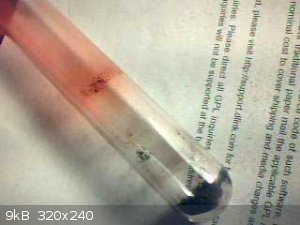
I ordered 10g. It arrived today. The first thing I noticed is the powder is low density not like mercury oxide. It is not soluble in water. On heating
about 100mg in an open test tube to a dull red temperature. The powder turned black and slight red condensation formed in the cooler part of the tube
with a thin dark red liquid line towards the heated portion of the tube. No sign of any mercury condensation.
I conclude the powder is not mercury sulphide
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Well, that's a few dollars wasted on my part. Thanks anyway, wg48, for the information.
The philosophy of one century is the common sense of the next.
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Quote: Originally posted by CobaltChloride  | I know that highly alkaline solutions damage glass frits from vacuum filtration systems. What is the maximum pH a solution can have without noticeably
damaging the frit?
[Edited on 15-3-2018 by CobaltChloride] |
Since nobody answered, I did some research. I found an article which says that porous glass is damaged by solutions with a pH >9.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
The item I purchased from Ebay was posted under this heading "Rocks, Fossils & Minerals > Mineral Specimens"
and described as "Natural Powdered Cinnabar Pigment"
I think I can reasonable claim it is not as described unless anyone knows a red mineral that turns black when heated and is slightly volatile. So I
should get my £1.49 back from Ebay or PayPal. If you purchased the same item you should get your money back too.
|
|
|
Volitox Ignis
Hazard to Self
 
Posts: 53
Registered: 28-1-2016
Member Is Offline
Mood: No Mood
|
|
Are alkyl halides acidic? If so, how strong?
|
|
|
OldNubbins
Hazard to Others
  
Posts: 136
Registered: 2-2-2017
Location: CA
Member Is Offline
Mood: Comfortably Numb
|
|
Quote: Originally posted by wg48  |
and described as "Natural Powdered Cinnabar Pigment"
I think I can reasonable claim it is not as described unless anyone knows a red mineral that turns black when heated and is slightly volatile. So I
should get my £1.49 back from Ebay or PayPal. If you purchased the same item you should get your money back too.
|
They also mention 'Curcuma' at the start of the description. Does it smell like tumeric?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
No, not in the Bronsted-Lowry sense. The can act as Lewis acids and be attacked by nucleophiles, but they can't really be deprotonated.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Another member had asked me by PM what is the best method to cleave an alkene in the presence of a benzylic methyl (thus without permanganate), if the
acid is acceptable. There are two methods I think are good: iodobenzenes/Oxone and tungstic acid/hydrogen peroxide.
Iodobenzenes/Oxone
These methods work by generation of an iodonium ion that cleaves glycols while being reduced to the iodobenzene, basically a recyclable periodate.
Various catalysts are used, including iodobenzene itself; iodomesitylene, which is quite good, may be available for purchase. I think the
easily-produced 3-iodobenzoic acid will suffice for most purposes, since you still generally save quite a bit of iodine vs. a periodate method:
Attachment: iodobenzoic_acid.pdf (242kB)
This file has been downloaded 475 times
A rough description of the method, using 4-iodobenzoic acid or iodobenzene in acetonitrile/water, is given here:
Attachment: iodoxy_olefinox.pdf (179kB)
This file has been downloaded 509 times
Since Oxone is responsible for glycol formation and iodobenzene is responsible for cleavage, I think that it might improve yields or lower catalyst
loading if you react the alkene with 1.1 equivalents Oxone for a few hours before adding the iodobenzene and remainder of the Oxone. Oddly none of the
papers I've read has actually tried this, though they all report the same mechanism I just described.
Oxidation with tungstic acid/hydrogen peroxide
A Japanese paper in 1989 reported that tungstic acid / hydrogen peroxide cleaves alkenes in refluxing tert-butanol. The only practical obstacle to
this method is obtaining tert-butanol, but I don't see why any solvent that dissolves the reactants wouldn't work. Acetonitrile/water in particular
seems like a reasonable bet. The reaction is sensitive to ambient acidity and works best in the range pH 4-5:
Attachment: tungstic_olefinox.pdf (320kB)
This file has been downloaded 482 times
Tungstic acid may be produced by dissolving tungsten in hydrogen peroxide, so the only thing you really need is tungsten... preferably in small
pieces, as the reaction is quite slow.
Oxidations with ruthenium or vanadium reagents are also effective for this transformation but are less OTC. N-hydroxyphthalimide catalyses a novel
photo-aerobic oxidation of certain highly activated alkenes, but not most of them. Periodate/osmate is a classic.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by OldNubbins  | Quote: Originally posted by wg48  |
and described as "Natural Powdered Cinnabar Pigment"
I think I can reasonable claim it is not as described unless anyone knows a red mineral that turns black when heated and is slightly volatile. So I
should get my £1.49 back from Ebay or PayPal. If you purchased the same item you should get your money back too.
|
They also mention 'Curcuma' at the start of the description. Does it smell like tumeric? |
No. The only smell from the open small plastic bag of the bright red powder that I could detect was a very weak smell I associate with plastic bags.
I also checked the smell and colour of some ground turmeric I have as a comparison. That has the earthy smell I associate with that spice and the
colour is very different and much less intense which I would describe as an orangey beige.
|
|
|
Volitox Ignis
Hazard to Self
 
Posts: 53
Registered: 28-1-2016
Member Is Offline
Mood: No Mood
|
|
Does a Grignard reagent have to be alkylmagnesium halide or can it be vinyl or alkyne-magnesium halide as well?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Vinyl will probably work. Allyl and aryl ones definitely do. Alkynylmagnesium halides would not be make by the reaction of a 1-haloalkyne with
magnesium, but by the reaction of a 1-alkyne with another Grignard (acid-base metathesis).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
..
81
82
83
84
85
..
104 |