| Pages:
1
..
6
7
8
9
10
..
20 |
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Lol I hadn't noticed. The blue blisterpack? thats mogadon (nitrazepam) and the brown bottle could be either one of two things, chlormethiazole
capsules or a few pieces of elemental silicon, since I end up with plenty of those amber glass bottles that the chlormethiazole comes in, its kinda
handy really since it can't be stored in plastic containers. At least, not if you ever want to get the chlormethiazole out again, it attacks plastic
pretty badly, although whatever the cap of the bottles it comes in are made out of survive it for at least a week. I think the box to the back of the
nitrazepam is pramipexole, one of the non-ergoloid dopamine receptor agonists with a strong bias towards DA-D3 agonism, also an agonist at D2
(primarily the short isoform, although still quite a strong partial agonist at D2l)
I take it for RLS. Don't know if you've ever had it, but RLS really sucks arse. Drives me up the bloody wall sometimes. If the box isn't that, its a
bit difficult to make out then its oxycodone IR, since I use it as a backup when the morphine or any improvements on it doesn't adequately make my
iffy joints and bursitis stop howling. Or possibly either clonidine or tizanidine, a couple of the imidazoline receptor agonist/alpha2 adrenergic
autoreceptor agonists, the former I use to prevent sensory overloading and the latter, as a muscle relaxant since having some surgery that A-didn't
help a damn and B-made things worse by inflicting some nerve damage that causes my calf muscle to be permanently contracted without it. Hurts like a
bitch, although the tizanidine does help a lot, and generally speaking the morphine/oxy or dipropionyl/dibenzoyl/dibutyrylmorphine/oxy combination
finishes off most of the aggro from the calf issue, aside from the paraesthesia and neuropathic type pain, for which opioids only really help when
either the dose is very large for the tolerance level of the person taking them, or else a pain med that really packs a punch is used (which is one
reason I favour the 3,6-dipropionyl ester. Lasts most of a day from a single IM or subcutaneous dose of around 600-700mg [tolerance, I'm not
suggesting anybody try it at that level, its perhaps as potent again compared to diamorphine as the latter is to morphine on a mg to mg basis, but for
some reason it lasts an awful long time)
(yes, I have the benzos, the chlormethiazole, oxy, morphine and the like on rx, not that such things are of much import, other than meaning I don't
need to result to synthesis myself)
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
It's interesting that your Thiele tube has a ground joint at the top.
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Do they not usually have one? its the only one I've actually seen right there in front of me.
I still need to read up on the use of them (in particular the flame-sealing of the capillaries is a little unclear, as to which (or both) ends are
meant to be sealed. Got plenty of tubes, a 100 pack was pretty cheap, a couple of dollars on ebay. And since they are disposable, I guess it matters
relatively little whether or not they happen to be made of chinesium.
IIRC the thiele tube was around $15-20, forget exactly but within that range give or take a dollar or two either way.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I've never seen one with a ground glass joint, but I haven't seen that many of them really, so perhaps they are common actually.
The capillary tubes with two open ends are kind of a pain to use. Typically you only need to seal one end.
I'm buying some more gas washing bottles (I swear you can't have too many). I should probably also get some U tubes in various configurations....
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Damn straight you can't. I've the one atm although will certainly be buying more dreschel bottles.
As for the ground glass joint, your in the same boat as I then only the inverse, since the thiele tube I own is the only one I've seen physically,
right in front of me as opposed to pics on ebay, and I more or less ignored the majority on price grounds and picked the one I could best afford. The
fact that it has a ground glass joint is simply a bonus. Although not entirely sure what use it could be put to, or why you'd attach a MP testing
device to a reaction setup when a capillary needs to be filled and sealed, strapped to a thermometer and the tube filled with oil or concentrated
H2SO4, I could see the utility in the joint if thiele tubes just took an internal sample, heated it and measured the MP of a dissolved solid, liquid
etc. But since the samples need the preparation that they do I'm at a loss as to why the joint is there, unless simply as a way of keeping it ready
and waiting to be used, clipped on to whatever there may bee by way of spare 24/40 male fittings.
That does seem an overly imprecise long shot though. You've gotten me curious now, wondering just why its there and what purpose the joint has. Could
it maybe be some kind of custom piece perhaps?
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Um...
Isn't the ground glass joint so that you can drop in your thermometer adapter with the thermometer in it?
As for the wash bottles, my latest purchase was this:
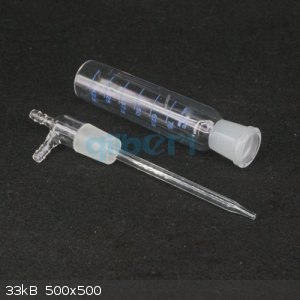
http://www.ebay.com/itm/25-125ml-Gas-Sampling-Tube-Glinsky-A...
Fine piece of glassware. The second I opened it I wished I had bought several.
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Should have thought of that. Serves me right for posting late at night the day before my scripts are due with all tanks running on fumes and being at
best somewhat scattered and shitty-feeling. I was envisioning simply placing the thermometer in the thiele tube without an adapter. Probably because,
aside from the ones attached to distillation adapters, my thermometer adapter is really short, in fact I've never used it, I've always used a still
head with a plastic stopper stuffed in the side-arm for temp. measurements, unless a flask is either really small or really full.
The arm part of the adapter is only a few inches long and actually its far more use as a cold-finger than it is for use as a thermometer adapter.
The lower piece of glassware, is that a thermometer adapter or a venturi-type aspirator vacuum pump? its difficult to tell if the bore narrows at the
point of the ground glass joint, although from the look of the top of it, with the wide hose barb I'm guessing aspirator vacuum. Different from mine,
I haven't seen one with a narrow taper like that at the very bottom. Mine has the taper in the bulb and a straight tube right to the end at the point
the water exits. Does that, assuming there is a taper in the point where the ground glass joint is also, improve the vacuum (the end taper that is) ?
Because if so, then it's time for toady to grab one of his blowtorches and make a little modification to his aspirator vacuum at the very end, so as
to be able to restore it easily if, on hooking the vac line to his vacuum chamber (although it'll take a long time to pump down with a venturi most
likely, considering the size of the thing) prior to heating and pulling down to a narrower tip then cutting the end with a diamond cutter or tungsten
carbide tool (actually an old cutter from the capstan lathe with a nick in the carbide tip making it no good for use in the lathe but its only a small
chip in one corner so its still ideal as a glass knife, which it has been repurposed for. Tsath's all for the waste not, want not mindset and recycles
as much as possible to be put to further use)
 
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Above is the vacuum chamber in question, the tall black thing with the 1/2'' or so thick acrylic (IIRC) windows top and bottom, couldn't easily get
the camera in a point to include the vac gauge and barb.
And a still flask (tubing attached), a bump trap, and a 10ml micro-flask.
Plus the long wire coil with a different sized brush at either end, its for cleaning flasks and the like as when pushed it coils up around inside them
to scrub off the typical o-chem related garbage that accumulates. The other pic, is a block of Na.
 
|
|
|
tsathoggua1
Hazard to Others
  
Posts: 335
Registered: 8-1-2017
Location: Beyond the pale
Member Is Offline
Mood: Phosphorescent
|
|
Oops, bad pic of the vac chamber. Better one:

|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Recently bought a 24/29 distillation apparatus and a 125 mL 24/29 separatory funnel in order to delve further into organic chemistry syntheses and
theory.
I also bought a 50 mL burette with a PTFE stopcock since I broke my ground glass stopcock for my 25 mL ground glass burette. I honestly prefer the
PTFE stopcock due to more of a smoother twisting and turning. I'm using this in my "real-life theory" journey where I explore the theoretical part of
chemistry. After all, theory before experimentation  . I could also use this for
some analytical chemistry I plan to study in the future. . I could also use this for
some analytical chemistry I plan to study in the future.
By the way, are there any uses for a burette without a stopcock?
[EDIT: added some missing prepositional phrases. My grammar is deteriorating  ] ]
[Edited on 10-12-2017 by ninhydric1]
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Just bought a 300mm glass vacuum desiccator.
Second hand, but no scratches or chips and it had a thick layer of drierite in it as a bit of a bonus.
Anybody know how hazardous it is to use one of these without a Desi-guard?
I've seen few Desi-guards in my life, so I sort of assume desiccators are pretty safe if you don't drop a brick on them when they're under vacuum or
something like that.
But what I don't know about vacuum implosion you could almost cram into the Astrodome.
|
|
|
Sulaiman
International Hazard
    
Posts: 3692
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
You can make the burette work again by cutting off the stopcock and adding an all ptfe stopcock.
Here is an overpriced example http://www.ebay.co.uk/itm/Detachable-Burette-Stopcock-/26323...
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
You can also attach a piece of hose and close it with a hose clamp. That's called a "pinchcock."
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by JJay  | | You can also attach a piece of hose and close it with a hose clamp. That's called a "pinchcock." |
Make it even better by adding a pasteur pipette to the end so that you vet nice controlled drips.
Incidentally my first condenser was made from a broken burette.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
Quote: Originally posted by JJay  | | You can also attach a piece of hose and close it with a hose clamp. That's called a "pinchcock." |
That's a good idea, but I just wish it didn't have such an uncomfortable name.
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Interesting uses. So I'll have a 25 and 50 mL burette now. That's nice.
|
|
|
18thTimeLucky
Hazard to Self
 
Posts: 51
Registered: 19-8-2017
Location: The one-and-only tea and crumpet land (UK)
Member Is Offline
Mood: 0 Kelvin and still won't crystallise from solution
|
|
Yay! Got some new glassware for my birthday along with a few other bits. This includes my first distillation apparatus so I am looking forward to
expanding into some organic chemistry. Most of it is lowish quality with a decent amount of bubbles but I am over the moon. Move out the way jam jars
- beakers and conical flasks here I come!
![IMG_7281[1].JPG - 2.1MB](http://www.sciencemadness.org/talk/files.php?pid=494703&aid=62002) ![IMG_7360[1].JPG - 1.8MB](http://www.sciencemadness.org/talk/files.php?pid=494703&aid=62004)
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I splurged and got a 1L fritted funnel in 24/40. I probably don't need one this big, actually, but hey, it should be just the thing for chromic
anhydride.

|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
A fused quartz jam jar jet.
"One of the most interesting of the system oscillations
is the Reynst pot phenomenon. The discovery of this
phenomenon was the result of an accident. F. H. Reynst
dropped a burning match into a near empty can of alcohol.
Instead of simply burning or going out, the can of alcohol
began to rumble at a low frequency. From this beginning,
pulsating combustion became the vocation and avocation to
which Reynst devoted his life." (page 18)
Naval Postgraduate Thesis by Robert Crow entitled Pulsating Combustion Device Miniaturization
http://www.dtic.mil/dtic/tr/fulltext/u2/a035707.pdf

[Edited on 24-10-2017 by Morgan]
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Some jars for Halloween
https://www.michaels.com/clear-skull-jar-with-lid-by-ashland...
Jam Jar Jet Skulls - If you freeze the clip at the 4 second mark there's quite a bit of green light saturating the frame. I only misted in a few shots
of boric acid methanol mix from a sprayer and the rest straight methanol so the effect wasn't as verdant.
https://www.youtube.com/watch?v=Z-oOYF7EDYk
Pergamon Press - Pulsating Combustion The Collected Works of F. H. Reynst
With a Jam Jar
http://www.pulse-jets.com/phpbb3/download/file.php?id=13590&...
[Edited on 25-10-2017 by Morgan]
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
Quote: Originally posted by 18thTimeLucky?  | Yay! Got some new glassware for my birthday along with a few other bits. This includes my first distillation apparatus so I am looking forward to
expanding into some organic chemistry. Most of it is lowish quality with a decent amount of bubbles but I am over the moon. Move out the way jam jars
- beakers and conical flasks here I come!
|
I remember my first flask and beakers. Good times...
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
My 100ml PTFE beakers arrived today about £4.50 each and free postage, excellent value. First test with a dye suggestes they are sintered well. HF
chemistry and boiling phosphoric acid now availble to me.

[Edited on 31-10-2017 by wg48]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by VSEPR_VOID  | Quote: Originally posted by 18thTimeLucky?  | Yay! Got some new glassware for my birthday along with a few other bits. This includes my first distillation apparatus so I am looking forward to
expanding into some organic chemistry. Most of it is lowish quality with a decent amount of bubbles but I am over the moon. Move out the way jam jars
- beakers and conical flasks here I come!
|
I remember my first flask and beakers. Good times... |
First ground glass was (probably) like a first kiss...
But I wouldn't know
|
|
|
18thTimeLucky
Hazard to Self
 
Posts: 51
Registered: 19-8-2017
Location: The one-and-only tea and crumpet land (UK)
Member Is Offline
Mood: 0 Kelvin and still won't crystallise from solution
|
|
Quote: Originally posted by The Volatile Chemist  | Quote: Originally posted by VSEPR_VOID  | Quote: Originally posted by 18thTimeLucky?  | Yay! Got some new glassware for my birthday along with a few other bits. This includes my first distillation apparatus so I am looking forward to
expanding into some organic chemistry. Most of it is lowish quality with a decent amount of bubbles but I am over the moon. Move out the way jam jars
- beakers and conical flasks here I come!
|
I remember my first flask and beakers. Good times... |
First ground glass was (probably) like a first kiss...
But I wouldn't know |
I can confirm. My girlfriend would not be surprised if I said this too her.
[Edited on 31-10-2017 by 18thTimeLucky?]
Yep, I have a chemistry blog!
18thtimelucky.wordpress.com
"Amateur chemistry does seem like being in a relationship with someone very beautiful and seductive but has expensive taste, farts a lot and doesn't
clean up after themselves, but you love them anyway" - a dear friend |
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Awhile back I bought this "LECO 550-122 C4303 Carbon Sulphur Analysis Glass Jet Combustion Tube - NOS" on eBay to tinker with.
It's made of quartz but I wasn't expecting the tiny hole in the transition, but still it's useful to me. There's 9 available if for some reason you
would want one.
  
[Edited on 31-10-2017 by Morgan]
|
|
|
| Pages:
1
..
6
7
8
9
10
..
20 |