| Pages:
1
..
6
7
8
9
10
..
13 |
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Antwain
Interesting thread. I have 1kg of bromochlorodimethlyhydantoin and want to make bromine from this.
The way I see it my options are throw in NaOH and boil it (slow addition may be better, not sure yet) to make NaCl:NaClO3:NaBr:NaBrO3 = 2:1:2:1. Then
slowly add HCl to the solution produced (minus the dimethylhydantoin, solubility 0.1g/100mL) while heating, to produce Br2 with perhaps some BrCl. Or
I could just add HCl to the BCDMH and get the bromine and halogens from that. If bromine is liberated first then the amount of HCl added could be
tailored so as to not liberate large quantities of chlorine.
Q1. Will concentrated, acidic halogens react with dimethylhydantoin to decrease my yield? ie. do I need to do an alkaline extraction first?
Q2. If the addition of HCl is slow will I be getting *mostly* Br2, BrCl, Cl2 or all of the above? If all of the above, then how the heck do I handle
100g+ of chlorine 
Q3. Since at least some BrCl will be formed how do I remove this from the Br2? I was thinking that by refluxing it up a column for ages I could let it
escape as Cl2, but using tap water to cool the column BrCl will probably be lost too (bp.'s of Cl2, BrCl and Br2 are -34*C, 5*C and 59*C
respectively). I am assuming that an equilibrium exists Cl2 + Br2 <---> 2BrCl.
Any help appreciated. 
[Edited on 7-10-2007 by Antwain] |
If you would have read the last two or three pages of this thread you would have seen all your questions were already answered.
According to the redox equation you need exactly 3/2 mol Na2SO3 for each mol of 1-bromo-3-chloro-5,5-dimethylhydantoin (in acidic media). But like it
was already explained, reducing 1-bromo-3-chloro-5,5-dimethylhydantoin with anything but KBr (or some other bromide) would be quite irrational as a
source of Br2.
Your idea of first "hydrolyzing" the bromochlorodimethylhydantoin by halate disproportionation in basic media followed by another disproportionation
in acidic media is a bit irrational to say the least. I see no rationale on why to do that. Not to mention that the first step would be terribly slow
since the bromochlorodimethylhydantoin with hydroxide is reversible with the equilibrium laying far to the left (thus the concentration of hypobromite
and hypochlorite would be very low at any given time).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Antwain, thats not the way to go about it. You can't separate bromine and chlorine by physical means. Use chemistry.
You can, for example, make use of the fact that H2O2 will liberate bromine from HBr, but not chlorine from HCl. So boil your BCDMH with NaOH, acidify
slightly, reduce both chlorate and bromate with SO2 (from K-metabisulfite), acidify strongly and add H2O2. Then only bromine will be set free. Distill
off. Free from chlorine traces by stirring with NaBr solution and distilling from it.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
But why? It simply does not make any sense when you can simply reduce BCDMH directly to Br2 and chloride ions. What role would boiling it with NaOH
have?
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
@ garage chemist- thanks, that seems like an acceptable way of doing things. Would you expect that 6% H2O2 would be up to the task or do I need to
find some 30%? Alternately, if i was to reduce some of it and then throw the mixture of MBr and MCl back in with BCDMH in the right proportions and
acidify with H2SO4 will this give me chlorine-free bromine? Since I believe that the answer to that is no, would the mixture that came across be able
to be reacted with some more mixture of alkali metal halides to produce only bromine?
@nicodem - I said help would be appreciated, so you can assume that your sarcasm is unappreciated. If MY questions had been answered then I would not
have asked them. That is all.
edit- a hydrolysis is probably not necessary in the case of a subsequent reduction. I only have a few grams of bromide and cannot easily get more
here. I was exploring other options, which have clearly lead to dead ends.
[Edited on 7-10-2007 by Antwain]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Sarcasm? WTF? Where? 
I answered your questions and even told you where you can find more detailed information. I even took time to calculate the amount of bisulfite
needed. For this I get to be accused of sarcasm? You really should think more about your ignorance and its origins.
Yes, your proposal for bromine generation is a great idea! (<- that is sarcasm from my side)
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
You are right, it is not sarcasm exactly, just general not-niceness. Whilst you clearly know what you are talking about, your post(s) have not helped
me at all towards my goal. Did you think that maybe if I am not going to use the bromide + BCDMH method which HAS been used successfully then maybe I
had a good reason, such as having no bromide. If you were trying to suggest to me that I could make bromide (which I could then oxidise with BCDMH) by
reducing BCDMH then you failed completely to communicate that idea in your first post. If that is not what you were suggesting then I still don't know
what you meant. It is one thing to tell someone that they are wrong, it is another to do so in a condescending and confusing way.
[Edited on 7-10-2007 by Antwain]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
If you didn't understood then say so. I will gladly explain if I can. But accusing me of sarcasm just because I tried to help you with a short post
instead of explaining basic redox chemistry in a lengthy post is not being nice from your side. Moreover, I directed you to other posts where you
could have found a lengthier explanation if you needed it!
Anyway, what I was saying in my reply is that if you do not want to use KBr as the reducing agent you can use sodium or potassium sulfite or bisulfite
in acidic media instead. This can come handy in cases where you can not obtain KBr (like in your case) but is not so rational chem-wise since you
would end up with less Br2. Another problem with the (bi)sulfite is that you need to use the exact amount needed to reduce BCDMH to chloride and Br2.
Using too much would result in reduced yields since Br2 gets reduced by SO2 as well. You would not encounter this problem if using KBr since even it
used in excess it can not reduce Br2, but utmost dissolve in the form of the unstable Br3(-) complex anions.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Nicodem is right with the equilibrium BCDMH + 2 NaOH <---> NaOBr + NaOCl + DMH being far on the left side. That would speak against the NaOH
treatment.
So direct reduction with 3/2 mol Na2SO3 per mol BCDMH would be much better, followed by acidification with HCl. If the amounts were right, only
bromine will be liberated... it would be better to use some excess of Na2SO3 and then oxidise the leftover bromide ions to Br2 with H2O2 after
acidification.
[Edited on 7-10-2007 by garage chemist]
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
If I did the sums right, a Kg of the hydantoin is about 4 moles. So you ought to get 2 mols of Br2 (four gram-atoms Br) out, or about 320 g. That is
only about 100 ml, it is dense stuff.
Don't throw away the dimethylhydantoin, it is easily regenerated to the haloamine.
Sic gorgeamus a los subjectatus nunc.
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
Those are the values I got too. I used Na2S2O5 as the reducing agent.
I am not sure that I will ever attempt this again. It was a complete failure (except that I got bromine). My lab book was destroyed in the chaos,
however I believe I used 254g of metabisulfite and 400 and something g of BCDMH. Either way it was correct to within 0.1g, 4:3 molar ratio.
I added some water to the 1L RBF connected to a 24/40 water condenser with a good flow and a 250mL RBF in an ice bath. The reactants were already in
it and had changed colour slightly to a slight red/orange.
The reaction started slowly and vapor was coming out the pressure vent, not much but it smelled. Then liquid started to condense in the condenser then
run into the flask. By now much vapor was coming out the vent so I figured that lightly placing something over it would help. It was not SEALED, but
still it blew out a plug on the 1L flask. Despite being emersed in a water bath it continued to react faster and faster. Somehow something blew out
(I don;t know what because I was unable to enter the shed by this point) but somehow bromine and or solution got into an oil bath. White fumes went
everywhere. Everything turned from bad to shit in an instant. I took a deep breath and grabbed some sodium sulfite that was very fortunately at the
very front of my cupboard (it was so white you couldn't see and it was a lachrymater). I turned the fuse off at the box and started spraying torrents
of water blindly into my shed then went in to get it to spray into the flask (better dilute on the walls than still reacting). This is when I got a
lungfull of something nasty. I threw the sulfite solution into the bromine which had distilled (it was full of water which had blown through the
condenser, but there was 20-40mL there even though I had added no acid). For the next half hour I was seriously contemplating going to the hospital
but decided that after half an hour the damage had been done. It probably isnt permanent but I still have a slight wheeze 
I don't know why it reacted so quickly and violently. Thank god my neighbors were still in bed cos you would have noticed this smell 100m downwind at
its peak. I have not been so scared in many years, especially when all hell had broken loose and I knew that the reaction was <10% complete and
when initially I was panicking and couldn't breathe at all. Thank god I had the sulfite at the front of the shelf, if it had been at the back things
would have been much worse.
I am at a loss as to the speed of the reaction. Earlier in this thread it was claimed that the reaction of BCDMH with bromide was not exothermic, so I
don't necessarily see why this should be. Maybe oxidising the metabisulfite made bisulfate, that would explain it (duh). In hindsight, adding the
metabisulfite in the form of solution, slowly would have been much smarter.
To be honest the main reason I am posting this humiliation is so I can tell you that if you are a n00b or a kewl or generally someone who knows
nothing about chemistry, then don't try this at home YOU WILL PROBABLY DIE!
IF, and thats a big IF, I ever try this again it will be scaled down several fold and there will be dry ice. I think I am going to take a break from
practical chemistry after this, at least until uni is over. Except tomorrow when I will have to spend 3 or so hours cleaning out my waterlogged shed,
waterlogged heating mantle, waterlogged graphics calculator. 
|
|
|
woelen
Super Administrator
        
Posts: 8085
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Antwain, sad to read about this (near) accident. Most important is that you personally have not been seriously injured. Please don't throw the towel
in the ring, I like your contributions and the things you manage to do, even in the difficult social climate of Australia.
Consider this event as a wise lesson. The goddess of chemistry is beautiful, but she must be handled with care and don't make her pissed off  . .
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Antwain, I'm sorry to hear about the disaster and wish you had no permanent lung damage or other consequences.
However, the kind of accident you had is a perfect example of malpractice. You actually did everything wrong in respect of preventing such a disaster.
So for the newbies who might do experiments with powerful oxidants I though to emphasize what is a definitive not to do:
1.) Never ever do exothermic reactions before testing them on a 10 mmol scale! When scaling up always consider that exothermic reactions do not scale
up smoothly at all.
2.) Never ever put the reactants together and then add the solvent. This will always cause a runaway even if the reaction is only slightly exothermic.
3.) At least one of the reagents needs to be in solution and the second one need to be added slowly in small portions/dropwise with temperature
monitoring and efficient cooling bath.
4.) If a reaction does not appear to start immediately do not heat or add more catalyst/acid/base or whatever the reaction mechanism requires to speed
it up. Have patience.
5.) Always use theory to evaluate the expected amount of heat or gasses evolved in the reaction and think of all possible influences any of the
parameters will have on the reaction proceeding and possible side reactions.
Edit: I forgot to mention that since HCl is one of the products the reaction actually needs no added acid to proceed to Br2. It is practically
autocatalytic.
[Edited on 7/10/2007 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
Yes I have embarrassed myself greatly with this one but fortunately after coughing up some questionable stuff my lungs appear to have returned to
normal. There was a reason why I chose to do it on this scale, although clearly it was a bad idea. The solubility of Na2S2O5 is not sufficient in
water for my to have completed this reaction in my largest vessel otherwise, and I was reluctant to take it apart and recharge it to complete the
procedure. I don't, in hindsight, think it was thermal runaway. I believe that it was agitation by gas causing greater mixing leading to greater
production of gas.
I will be out of the lab for the next several weeks as I do not have the time anyway, but I will be back after that. Ok, lets suppose I want to do
this properly, later. I am now thinking that running metabisulfite into hot but not boiling, wet BCDMH may be bast. The reaction will be quick at that
temperature, and the rate can be moderated by addition of reducing agent, with external cooling with a water bath if the mixture heats up or heating
with a mantle if it is not exothermic enough. This can then pass through a condenser to a large enough flask immersed in ice water, possibly with a
few mL of H2SO4 in it. The gas outlet from the condenser can be put through tubing (which will be attacked but not destroyed) and then into a sintered
bubbler ( I need to get one anyway really) which will bubble through sodium sulfite or metabisulfite solution. Do you see a problem with this method
nicodem?
Also, Whilst I was able to come up with the ratio BCDMH : Na2S2O5 = 3:4 myself, I couldn't balance the hydrogen ions needed and liberated, and I
didn't account for the fact that oxidising Na2S2O5 + H2O + 2[O] ---> 2NaHSO4 which is acidic! can you help me with the FULL balanced equation for
this. Or even just tell me that I do not need to add any acid which would be as useful. Thanks. Actually one more thing... If it makes HCl, should I
be running a stoichiometric mixture of metabisulfite and NaOH into the solution to prevent this from distilling over as well, or will this also
prevent the reaction from working.
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
To produce large ammounts of bromine with ease i use following reaction:
5KBr + 3H2SO4(aq.) + KBrO3 => 3Br2 + 3K2SO4 + 3H2O
Procedure is straight forward: 63g KBr is dissolved in 300 ml of water, 18 ml of 95% H2SO4 is added with stirring (car battery acid can be used to
disslolve KBr, taken in such ammount that resulting H2SO4 concentration is about 10%). Solution is transfered to 500 ml flask and 17.5g of potassium
bromate is added by small portions with stirring. Stirring is continued until large drop of liquid bromine is formed in the bottom. Bromine is
separated on separating funnel and dried with concentrated H2SO4. Yield is almost quantative, but some ammount of bromine remains dissolved in water
(it is not large and depends from temperature).
Photo with bromine made by this reaction is shown below:
[Edited on 12-10-2007 by Engager]

|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
Well if I had KBr, and KBrO3 instead of neither, then I am sure that is the method I would use 
|
|
|
Siddy
Hazard to Self
 
Posts: 81
Registered: 8-10-2007
Member Is Offline
Mood: No Mood
|
|
A percent yield for that post would be nice.
Also, where can you buy potassium bromate?
haha, guy above beat me.
[Edited on 11-10-2007 by Siddy]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
"Almost quantitative" = 90% range.
|
|
|
woelen
Super Administrator
        
Posts: 8085
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
| Quote: | Originally posted by Siddy
A percent yield for that post would be nice.
Also, where can you buy potassium bromate?
haha, guy above beat me.
[Edited on 11-10-2007 by Siddy] |
Potassium bromate you can make yourself. I posted a thread about this on sciencemadness. For your convenience I will post the method here. For
comments and further discussion of this, please search the forums.
http://woelen.scheikunde.net/science/chem/exps/KBrO3_synth/i...
Using KBrO3 and KBr for making Br2 indeed is very nice. This reaction runs very smoothly, it only is slightly exothermic and has very good yield. The
only issue is that quite some bromine may remain dissolved in the water, this has to be taken out by distillation.
I expect that engager will have a yield of approximately 75% if he only took the blob of liquid bromine and did not distill the bromine, dissolved in
the water.
Solubility of bromine in pure water is appr. 3 gram per 100 ml. When H2SO4 is dissolved, and no excess bromide is present, then it is somewhat less.
[Edited on 12-10-07 by woelen]
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
| Quote: | Originally posted by woelen
I expect that engager will have a yield of approximately 75% if he only took the blob of liquid bromine and did not distill the bromine, dissolved in
the water.
[Edited on 12-10-07 by woelen] |
Yes, you are correct. Yeild is near to quantative then bromine dissolved in water is recovered. I usualy do this after large ammount of bromine water
is accamulated, from several preparations of bromine. By the way the byproduct - bromine water is quite usefull for many experiments, such as
preparation of CBr4 from acetone by action of hypobromite (made by dissolving NaOH in ice cold bromine water), or preparation of isocyanogen
tetrabromide from sodium 5,5'-azotetrazolate. So, don't waste bromine water it can be quite usefull.
[Edited on 12-10-2007 by Engager]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Antwain
Also, Whilst I was able to come up with the ratio BCDMH : Na2S2O5 = 3:4 myself, I couldn't balance the hydrogen ions needed and liberated, and I
didn't account for the fact that oxidising Na2S2O5 + H2O + 2[O] ---> 2NaHSO4 which is acidic! can you help me with the FULL balanced equation for
this. Or even just tell me that I do not need to add any acid which would be as useful. Thanks. Actually one more thing... If it makes HCl, should I
be running a stoichiometric mixture of metabisulfite and NaOH into the solution to prevent this from distilling over as well, or will this also
prevent the reaction from working. |
The reduction should be carried by carefully adding BCDMH in small portions into a stirring, less than 10%, solution of the sulfite (for the exact
amounts see the reaction stoichometry) in a flask immersed in an ice bath. Since the reaction media gets more and more acidic it is best to use Na2SO3
instead of bi- or metasulfite to yield the neutral Na2SO4 instead of acidic NaHSO4. This is only important as to prevent too much SO2 escaping from
the reaction mixture thus ruining the exact stoichometry. It is therefore advised to add 1/2 of Na2CO3 per every NaHSO3 used if one has no Na2SO3
available. It is also important to add BCDMH to the sulfite as the opposite order gives rise to Cl2 formation (before all BCDMH is reduced to
1-bromo-DMH, there is Cl2 equilibrating in the reaction mixture).
Bellow are the relevant redox reactions (I hope I calculated all the electrons correctly, otherwise someone please correct me).
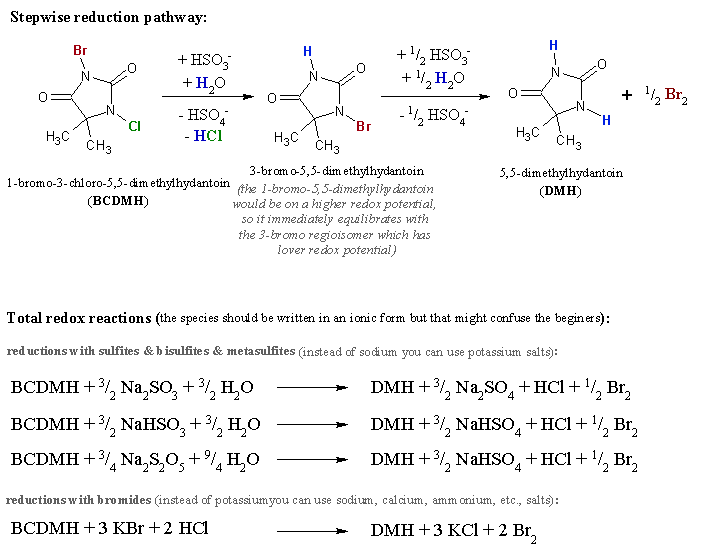
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
@Nicodem- thanks for that. I was concerned about the acid, but it completely escaped me that I would be losing SO2 as well. At 0*C will the bromine
have a low enough vapor pressure for this reaction to be done outside a fume hood, of course the BCDMH will have to be added slowly to prevent the
temperature rising.
|
|
|
Antwain
Hazard to Others
  
Posts: 252
Registered: 21-7-2007
Location: Australia
Member Is Offline
Mood: Supersaturated
|
|
I couldn't stay away from the lab. I dissolved 94.5g of Na2SO3 in ~400mL of water and cooled in ice water. I added added 121.6g of BCDMH in small
portions with stirring. During this time 24 ice cubes were consumed (not a good measurement, but it gives some idea), and a further 12 ice cubes were
melted in the following few minutes. At all times there was spare ice in the bath.
I didn't want bromine liquid per se, so a solution of 28.4g of KOH in ~150mL of water was prepared in a Erlenmeyer and a liebig was set up with a
small length of disposable plastic tube to dip into the flask. For a long time only vapour came across but when i ramped the temperature up finally
bromine started condensing. The tube was attacked and discoloured, but this was not a concern and it seems that the very small amount of crap will be
able to be filtered out. Heres the shit part.... Not only did not all of the bromine distill across (most of it came) and the reaction flask is still
somewhat red, but also the collection flask had to have extra KOH added (a few g) to stop it from turning polyhalide coloured and smelling bad. I
should also point out that after bromine stopped distilling across (at a temp of 58-63*C) I had to remove the thermometer so I could stick a pipette
connected to an air pump down and put roughly a bubble a second through the flask to purge the vapour above the solution. This did get much more of
the red colour out. So.... Not all the bromine distilled and yet the base was inadequate.... Clearly HCl came across too.
Can someone tell me why my bromine was so reluctant to leave solution, and suggest a modification to fix it next time. Also it may be prudent to
collect liquid bromine next time, possibly beneath H2SO4, so that all the HCl buggers off.
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
I too have extracted bromine in dilute form the electrolysis of NaBr in a separatory funnel. I plan to distill it with H2SO4 to remove water and
condense pure bromine, though I am not sure of the exact composition of the liquid precipitate I got. I guess it is just dilute bromine/hypobromous
acid.
Anyway, more recently I have thought of a method using a rather well known set of reactions to obtain any of the halogens except fluorine in highly
purified form with very easy decompositions. By adding sodium bromide to a solution of silver nitrate or sulfate (the latter would be less preferred
due to its very low solubility, but it could work especially if you have trouble getting/making nitric acid), one can precipitate very pure silver
bromide (or chloride or iodide). As the silver halides are photosensitive and decompose to pure silver and halogen, the halogen can be isolated from
the decomposition flask with a simple distillation. The silver powder left behind can be easily acidified and reused as silver is an expensive metal
and will likely be in limited quantity
NaBr + AgNO3 --> AgBr + NaNO3
2AgBr -(light)-> 2Ag + Br2
The recycling of the silver and also the nitrate (or sulfate) ion could likely be simplified by using copper bromide as an intermediate, as the copper
salts are easily regenerated to acid.
NaBr + H2SO4 --> NaHSO4 + HBr
2HBr + Cu -(H2O2)-> CuBr2 + H2
CuBr2 + 2AgNO3 --> 2AgBr + Cu(NO3)2
2Cu(NO3)2 -(170°C)-> 2CuO + 4NO2 + O2
4NO2 + O2 + 2H2O --> 4HNO3
HNO3 + Ag --> AgNO3
CuO + 2HBr --> CuBr2 + H2O
repeat CuBr2 + 2AgNO3 --> 2AgBr + Cu(NO3)2
Alternatively:
CuBr2 + Ag2SO4 --> 2AgBr + CuSO4
CuSO4 -(650°C)-> CuO + SO3
SO3 + H2O --> H2SO4
H2SO4 + 2Ag --> Ag2SO4
CuO + 2HBr --> CuBr2 + H2O
repeat CuBr2 + Ag2SO4 --> 2AgBr + CuSO4
The mind cannot decide the truth; it can only find the truth.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Antwain
Can someone tell me why my bromine was so reluctant to leave solution, and suggest a modification to fix it next time. Also it may be prudent to
collect liquid bromine next time, possibly beneath H2SO4, so that all the HCl buggers off. |
Quite surely some HCl distilled over, but that might not have been the main reason your 28.4g KOH did not suffice to trap all Br2. It appears to me
that you have not considered that KOH pellets are only about 85% KOH with the rest being water (the amount you used is actually 0.43 mol and not 0.5
mol as you intended). You would actually need at least 33g KOH for complete neutralization. Also, it is very difficult to distill or extract
all bromine from water solutions due to various equilibriums and interactions in the system (bromine disproportionations, bromide/bromine
complexations and DMH+Br2 reversibility). There will always remain enough for the reaction mixture to remain bromine-like colored. Just ignore that
and judge its presence by the vapors color instead.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
The decomposition of silver halides is neither fast nor complete. Plus unless you have the halide as extremely small particles the outer layers will
shield the inside of the halide particle from light.
If you have simple halide slats, such as NaBr, to start with, just use the halide salt + MnO2 + H2SO4 distillation to obtain the free halogen. Even
low grade MnO2 will work, if it's from old batteries just wash it well with water to remove chlorides. This eliminates the problems with recovering
bromine from water solutions.
In the case of organic sources such as spa "bromine" tablets, then an intermediate isolation step is useful.
An very old industrial method used to recover dilute bromine vapours was to absorb in alkali solution, evaporate to dryness and then heat strongly to
decompose bromate, then extract with methanol which left most of the chlorides behind. The extraction trick works even better with NaI, for which
acetone can be used as a solvent.
To remove chlorine from bromine or iodine, or bromine from iodine, distilling the halogen from some of its sodium or potassium salt will do the job.
The salts remaining behind can be repeated used until they are mostly the chloride, at which point the extraction with methanol can be used to recover
the last of the bromide or iodide from the chloride.
|
|
|
| Pages:
1
..
6
7
8
9
10
..
13 |