| Pages:
1
..
6
7
8
9
10
..
33 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Related to the shock/friction question testing question above-
Have any members gone so far as to MAKE either a drop hammer or friction shoe apparatus? On a quick search, I find only 1 EM thread regarding this?
http://www.sciencemadness.org/talk/viewthread.php?tid=26165#...
Likely several professional members have equipment at work, but do any "advanced amateurs" bother to?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hennig Brand  | | Thank you. So far I haven't done much for friction and impact testing, but I can tell you it takes a harder blow to set off DDNP than the other
primaries I have tested such as lead azide, silver azide, lead styphnate, mercury fulminate, TATP, HMTD, etc. I haven't used a proper testing
apparatus to accurately quantify the difference, but I can say that even with a crude hammer test it is obvious that DDNP is significantly less
sensitive to impact than most other primaries commonly used. Crude tests with hammer on concrete indicate that it is relatively insensitive to
friction as well. |
Thanks. Its interesting information, even the primitive tests conditions. Maybe I’ll give a try to DDNP someday. I think a mixture of DDNP and
around 20 – 30 % SADS will be something worth to try.
Anyway. Very good job and thanks again for the nice reading from you and Rosco.
|
|
|
Jimbo Jones
Hazard to Others
  
Posts: 102
Registered: 15-10-2009
Member Is Offline
Mood: No Mood
|
|
Yes. AXT have done it.
http://www.youtube.com/watch?v=_Xx-zhALHjM
http://www.youtube.com/watch?v=PAuzMOd9L48
I also made similar, but more simple rig years ago. It’s easy to assemble even with almost now tools.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
2g of Picric Acid Initiated in 7.6mm id Al Tubing by 0.25g DDNP & 0.05g of Lead Azide Hand Pressed in Steel Reinforcing Cap
This test was performed with the same materials and in the same way as the last test except that 0.05g of the 0.30g of DDNP was replaced with lead
azide. The 0.05g of lead azide was hand pressed into the reinforcing cap first followed by the 0.25g of DDNP. Lead azide, being a more instantaneously
detonating or unequivocal primary explosive, made the transition from deflagration to detonation very quickly thereby eliminating the longer run up
distance that is needed when DDNP is used alone. It is obvious from looking at the witness plate shots that the picric acid was driven much harder in
this test than in the last. The hole produced in this test was not quite as large as in some of the tests where more DDNP was used, but it looks as
though very little more picric acid output would be needed to produce similar results as was seen in those previous higher output tests.
The next experiment will be to form a small amount of silver azide on the surface of a stirred suspension of DDNP crystals. Apparently very small
amounts of silver azide formed on the surface of sluggish primaries can help them make DDT much more quickly.
Yes, as long as SADS is compatible with DDNP it would probably be a good paring too.
The holes on the right, in the witness plate shots, are from this test.
  
[Edited on 24-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
historical variations as alternative methods
There is a patent US1460708 which describes a variation of a much older method of producing DDNP reported by Stenhouse in 1868, both references
attached.
Attachment: US1460708 process for DDNP.pdf (319kB)
This file has been downloaded 763 times
Attachment: Pages 150-151 re DDNP Vol 21 (1868) Journal_of_the_Chemical_Society.pdf (144kB)
This file has been downloaded 946 times
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Does someone know if the following do exist?
-Diazo-dinitroaniline DDNA (or DNBTA dinitrobenzotriazole)
-Di-diazodinitrohydroquinone (DDDNHQ)
-Di-diazodinitroresorcinol (DDDNR)
-Dinitrobenzobistriazole (DNBBTA)
-Dinitrodiazoxybenzotriazole (DNDBTA)
Hereunder molecular structures vs related DDNP.
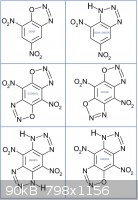
All those should display good densities and detonic properties although the di-diazoxy compounds might be very sensitive probably more than the DDNP.
The tricyclic compounds will be denser than the initial DDNP (d=1,63 (max density of bulk), d=1,71 (cristal)).
Note that the molecules containing a triazo ring hold also an acidic H that would allow to make energetic amine salts (NH4, N2H5 or NH3OH) or
energetic metalic salts.
The anionionic result of those are fully aromaticized what is good at increasing the density.
Those triazo ring are maybe in equilibrium with azido benzene structure what are known to be relatively stable if an ortho nitro group is present
(under heating those are oxydo-reduced to di-nitroso (furoxan) compounds.
-C(-NH-N=N-)C- <==> -CH=C(-N3)-
-C(-NO2)=C(-N3)- <==> -C(-N=O)=C(-N=O)- + N2
-C(-N=O)=C(-N=O)- <==> -C(=N(O)-O--N=)C-
This is good for thermal stability.
The acidic hydrogen atom can switch to the opposite nitrogen of the same triazole ring directly in contact with the aromatic carbon.
-C(-NH-N=N-)C- <==> -C(-N=N-NH-)C-
So two apparent different molecules are in fact identical (this effect is not observable with the diaza-oxy heterocycles.
[Edited on 8-5-2014 by PHILOU Zrealone]
Also what about detonic properties of Iso-diazodinitrophenol (IDDNP) (see picture hereunder vs usual DDNP)?
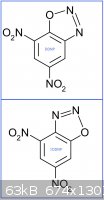
[Edited on 8-5-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
This is not exactly what you are asking about but seems somewhat related and interesting
Attachment: Diazidodinitrohydroquinone H-228 from Vol. 7 H-L.pdf (88kB)
This file has been downloaded 854 times
Attachment: GB207563 Diazidodinitrohydroquinone.pdf (371kB)
This file has been downloaded 694 times
[Edited on 8-5-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
2g of Picric Acid Initiated with 0.4g of DDNP & 0.1g of Lead Azide (Unreinforced Configuration)
This test involved the initiation of 2g of picric acid without a reinforcing cap. Two grams of picric acid was incrementally lever pressed into a
7.6mm aluminum casing followed by 0.4g of DDNP (hand pressed) followed by 0.1g of lead azide (hand pressed). Visco fuse was used as a delay element
and the end was sealed with hot melt glue.
There was a complete detonation but a clean hole was not blown through. I now believe that this is because of not pressing the picric acid base charge
to as high a density as in some of the early tests. I had to change the loading dowel that I was using a while back and in doing so created a
situation where there is less mechanical advantage obtained from the lever press. Another test will need to be performed with the picric acid base
charge at a loading density similar to the early tests.
The holes on the right, in the witness plate shots, are from this test.
  
BTW, I tried coating 4g of DDNP with silver azide so as to make a primary explosive composed of 5% silver azide and 95% DDNP. Previously prepared DDNP
was put into a well stirred suspension in water with the correct quantity of dissolved silver nitrate. On adding the sodium azide solution, slowly
with a pipette, effervescence was seen. I guess the solution was acidic; there could very well have been significant amounts of picramic acid
contamination with the DDNP especially since the sample of DDNP used was not recrystallized.
[Edited on 11-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
2g of Picric Acid Initiated with 0.4g of DDNP & 0.1g of Lead Azide (Dry Mixed Primary & Unreinforced Configuration)
This test involved the initiation of 2g of picric acid without a reinforcing cap. Two grams of picric acid was incrementally lever pressed into a
7.6mm aluminum casing followed by 0.4g of DDNP & 0.1g of lead azide which were dry mixed before being hand pressed into the detonator casing.
Visco fuse was used as a delay element and the end was sealed with hot melt glue.
As in the last test, there was a complete detonation but a clean hole was not blown through. I now believe that this is because of not pressing the
picric acid base charge to as high a density as in some of the early tests. I had to change the loading dowel that I was using a while back and in
doing so created a situation where there is less mechanical advantage obtained from the lever press. Another test will need to be performed with the
picric acid base charge at a loading density similar to the early tests.
The holes on the right, in the witness plate shots, are from this test.
  
[Edited on 11-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
A safety related point: Hot melt glue is... Hot.
There have been deaths associated with use of hot melt adhesives on commercial assembly lines for consumer pyrotechnics in USA manufacturing plants.
While there ARE low temperature adhesives and thermostatically controlled glue systems available, I'd still be reaching for the 5 minute epoxy if
sealing a metal device with live material installed. Just not worth adding another potential ignition source for a bit of speed/convenience!
And if it's a cheap consumer grade tool from the hardware or craft store- Well, read this:
http://www.amateurpyro.com/forums/topic/3530-hot-glue-guns/
http://www.lawyersandsettlements.com/news/02336/Glue-Gun-Fir...
[Edited on 11-5-2014 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I use the low temperature variety and push in a little wad of tissue in the casing over the primary and beside the fuse before applying the glue. I
also clean the inner and outer surfaces of the cap casing end as much as possible before gluing as well. I have always suspected that it was a bit of
a risk though. For instance if a glue gun malfunctioned and was hotter than normal it could have dire consequences.
Thanks for the concern and the links. Unplugging the glue gun before use is a good idea, and is one which I have used in the past.
[Edited on 11-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
For the integration of silver azide and DDNP a different scheme may be better.
What I think could work is form the silver azide separately as a colloidal or somewhat larger form and substantially dry it and then suspend it
stirred rapidly in warm toluene. Dissolve the DDNP in warm acetone and add that gradually to the stirred suspension. A small and very gradual
addition of water should complete the precipitation of the DDNP. Any trace acidity in the DDNP could be neutralized with ammonia or ammonium acetate
or carbonate.
It would probably serve even better to divide out a portion, maybe a third of the silver azide and add it last to the stirred suspension, to
accomplish external as well as included particles of the silver azide with the DDNP. Some of the graining additives like PVA and a surfactant could
be helpful also in the agglomeration of the particles as a density improver.
[Edited on 11-5-2014 by Rosco Bodine]
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
How about dissolving some Viton in that acetone? Poly vinyl alcohol is water soluble, as I recall it resists organic solvents very well?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Stearic acid, ester gum, microcrystalline wax, shellac, red gum, rosin, even latex or acrylic aqueous colloidal suspension paint component, and some
urethanes soluble in acetone like aluminum marine grade "trailer kote" polymerize anerobically on exposure to moisture ...there are many possible
candidates for trace adhesive tackiness as a graining additive and density improver even in very small amount ranges like a few hundreths of a per
cent impurity. Red goo is always good, works for everything 
Edit: linseed oil is another possible tackifier
Another potentially better scheme for coating the DDNP with silver azide could make use of the stability of DDNP to acetic acid. Acetone and acetic
acid could be used to dissolve DDNP and then an aqueous ammonia solution of Silver Azide
sould be added. The neutralization of the ammonia by the acetic acid would cause the dissolved silver azide to preciptate, and likewise the moisture
from the aqueous solution of silver azide would cause precipitation of the DDNP. Either order of addition should work but one or the other may
produce a better result. I tend to favor the order of addition that would be adding the ammoniacal aqueous solution of silver azide to the warm
acetone and acetic acid solution of DDNP. A milder less pH extreme variation technique would be concurrently adding the acetic acid and the
ammoniacal solution of silver azide as parallel additions to the warm acetone solution of DDNP.
[Edited on 11-5-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
2g of Picric Acid Initiated with 0.4g of DDNP & 0.1g of Lead Azide (Dry Mixed Primary & Unreinforced Configuration)
I got the density of the picric acid base charge back up to more or less what it was in earlier tests, other than that this test was the same as the
last test. As expected the higher density picric acid detonated with higher velocity and caused more damage to the steel witness plate on detonation.
A hole was blown through, but was not really a clean hole. The reinforcing cap from earlier tests may have not only had a lot to do with the higher
efficiency of the DDNP primary but also the picric acid base charge.
What I need is a gauge of some sort so that loading pressure can be easily monitored during cap loading. I guess a set of bathroom scales can be made
to work in a sort of improvised arrangement.
The holes on the right, in the witness plate shots, are from this test.
  
Regarding silver azide and DDNP mixtures, I am not all that familiar with some of the things you are proposing, but it sounds interesting. How is ASA
made? I always assumed that the lead azide, lead styphnate and aluminum powder were dry mixed or damp mixed, but I never really knew for sure what was
actually done.
[Edited on 12-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
What exactly is the mil spec standard ASA mix I am not certain. I think the ASA is a solvent damp mix using a bit of gum or wax or other material as a
binder and pressure flowable sealer. Even a small percentage of TNT can be used as an additive there and to help seal the pressed composition. The
fine flake aluminum also serves as a physical barrier like shingles on a roof so the mixture is pretty well sealed as a compressed pellet and is
storage stable.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: |
What I need is a gauge of some sort so that loading pressure can be easily monitored during cap loading. I guess a set of bathroom scales can be made
to work in a sort of improvised arrangement.
|
A cheap 1 ton arbor press from Harbor Freight or similar (Canadian Tire?):
http://m.harborfreight.com/1-ton-arbor-press-3552.html?utm_r...
A torque wrench in place of the supplied handle:
http://www.amateurpyro.com/forums/topic/8695-arbor-press-tor...
A load cell/pressure gauge to calibrate if needed:

AND!!!
A blast shield between you and the pressing operation- You can only stay lucky so long...
https://www.sciencemadness.org/whisper/viewthread.php?tid=30...
[Edited on 11-5-2014 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for the good suggestions. Yes, it is true, you can only stay lucky so long. There will always be risk, but the idea should be to try and keep
it to a minimum, while at the same time not being paralysed by fear to the point of not being able to try new things.
I like the arbor press idea. I almost got a good second hand one a while back, but as you pointed out they are not necessarily that expensive new
anyway. I took a picture of the pellet press we were using to prepare fuel samples for a bomb calorimetry lab. I think one of these would be fairly
easy to build from junk yard steel and would work quite well. A larger one than is shown in the picture might be more desirable for our purposes. The
pressing surface, where the die sits, is threaded and can be adjusted for height by turning it. There is a spring in the body of the press that places
enough upward pressure on the press plunger to keep it in the upward position until the lever is pushed down.

Found this patent which gives a little background information regarding the lead azide, lead styphnate & aluminum powder primary explosive mixture
ASA.
Attachment: Patent Regarding ASA.pdf (401kB)
This file has been downloaded 950 times
[Edited on 11-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Just want to point out that silver nitrate or other salts are usually uncompatible with ammonia... so Rosco's idea is rather dangerous!
silver nitride/amide
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Have you read the literature regarding silver azide manufacture? I should post a few references about silver azide manufacture processes in the
azides thread. The use of ammonia has been common for more than 50 years.
I'll just post the references here, maybe link em later there.
This thread and the associated picramic acid thread are likely headed for being stickied and combined somehow since the topic has become authoritative
as a reference.
Attachment: Silver Azide Pages from Technology of the Inorganic Azides, Vol. 2 (1977).pdf (1.1MB)
This file has been downloaded 1388 times
Attachment: US3943235 Process for Silver Azide Costain.pdf (449kB)
This file has been downloaded 737 times
Attachment: Silver Azide preparation a132994.pdf (1MB)
This file has been downloaded 1186 times
Attachment: GB887141 Imrovement in silver Azide.pdf (188kB)
This file has been downloaded 936 times
Attachment: GB781440 Improvements in Silver Azide manufacture.pdf (378kB)
This file has been downloaded 810 times
[Edited on 13-5-2014 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
British Mil Spec Silver Azide RD1336
Attachment: Silver Azide RD-1336.pdf (1.9MB)
This file has been downloaded 920 times
http://translate.google.com/translate?depth=1&hl=en&...
Attachment: Exploders Google Translate AgN3.pdf (8kB)
This file has been downloaded 926 times
[Edited on 13-5-2014 by Rosco Bodine]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Rosco Bodine  | Have you read the literature regarding silver azide manufacture? I should post a few references about silver azide manufacture processes in the
azides thread. The use of ammonia has been common for more than 50 years.
I'll just post the references here, maybe link em later there.
This thread and the associated picramic acid thread are likely headed for being stickied and combined somehow since the topic has become authoritative
as a reference.
[Edited on 13-5-2014 by Rosco Bodine] |
I stand corrected  . .
In one of your reference they mention that there is indeed a risk and a safety concern about Ag nitride/amide; but that the heating/venting (exhaust
of all the NH3 from the media) and that the neutralisation by nitric acid (or acetic acid) both allow the nearly complete conversion of the silver
into AgN3 and avoid further problems with Ag3N, Ag2NH and Ag-NH2.
On another point ammonia and aceton form a Schiff's base (*) what might (or might not) be a problem; your procedure must be tried in tiny amount
first.
(*)
(CH3-)2C=O + NH3 --> (CH3-)2C=N-H + H2O
On standing (for weeks) it turns into dark orange-red-brown polymeric material with a phenolic smell.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yeah ammonia and ammonium acetate increase greatly the solubility of azides including also lead azide and this may be helpful to solubility in the
azo-clathrate formation but I haven't tried the technique to confirm. Silver fulminate is also desensitized by ammonia forming a complex which can
allow its safe handling as the ammonia complex, from which the ammonia volatilizes and escapes gradually on drying and leaves the uncomplexed silver
fulminate as its usual sensitive form. I think IIRC it was Gerald Hurst patented that ammonia complexation process for silver fulminate, so there is
a parallel history for use of ammonia even with actual fulminate, under conditions which may still present the goblin possibility of formation of the
unwanted "fulminating silver" nitride. Process control is everything there for avoiding unpleasant surprises.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
This file has already been attached in the azides thread, but it seems important enough to put here also. In this report they examined a number of
different sensitizers for silver and lead azide. The good news is that DDNP seems to be compatible with both lead and silver azide. The bad news is
that DDNP seems to be a very effective sensitizer of both lead and silver azide. I would need to look into it further, but the stab initiation
energies table on page eighteen shows extreme sensitivity increase when DDNP is added to either lead or silver azide. In fact, of the handful of
sensitizers they tested, DDNP was the second most effective sensitizer in both cases.
Nice to know lead and silver azide are compatible with DDNP, but I think it might be best to just put a little azide on top of a DDNP charge rather
than mix the two. I suppose this could be what some of those references meant when they stated that lead azide and DDNP were incompatible; kind of
like when safety sheets say ammonium nitrate and fuel oil are incompatible.
File can be viewed here.
http://www.sciencemadness.org/talk/viewthread.php?tid=1987&a...
[Edited on 16-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I just posted that Australian reference above. I would disagree about the sensitivity because what is being described is specifically a particular
type of sensitivity to a compressed pellet being pierced by a sharpened firing pin called a "stabber" or a stab detonator. The compatability of DDNP
with lead azide was such that it may adversely affect the storage life of the lead azide, not that it causes any dangerous sensitivity. And the same
thing is reported about the ASA mixture being an incompatible mixture of lead styphnate with lead azide because of an increased decomposition rate of
a mixture of lead azide with lead styphnate that reduces the storage life. But as a practical matter the advantages of the ASA mixture outweigh the
slight reduction of the storage life for pure lead azide so ASA is used anyway. What is the real compromise if the expected storage life for an
ordnance is reduced from a predicted 300 years to only 150, as an example only for the sort of difference or storage life penalty which they are
describing when they speak of the incompatability which is more likely limited for the mixture by the DDNP storage stability anyway. The more ideal
storage life for the single component is better than for the mixture. But the difference is not so great over any practical storage time expected
that
it would be a real concern that would rule out the use of such mixtures.
[Edited on 16-5-2014 by Rosco Bodine]
|
|
|
| Pages:
1
..
6
7
8
9
10
..
33 |