| Pages:
1
..
6
7
8
9
10
..
23 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Yeah 2,4,6-tricarboxymethyl-1,3,5-triazine would be interesting to test although the resulting tris-nitroformyl triazine can be acheived by easier
ways (*).
Cl-CH2-CO2H + Na-C#N --> N#C-CH2-CO2H + NaCl
3 N#C-CH2-CO2H -trimerization-> cyclo(-N=C(-CH2-CO2H)-)3
cyclo(-N=C(-CH2-CO2H)-)3 -nitration-> cyclo(-N=C(-C(NO2)3)-)3
As sugested in http://en.wikipedia.org/wiki/2,4,6-Tris(trinitromethyl)-1,3,5-triazine
(*)By metathesis like it is the case for cyanuric triazide (cyanuric chloride and NaN3)...
cyclo(-N=CCl-)3 + 3NaC(NO2)3 -solvent-> cyclo(-N=C(-C(NO2)3)-)3 + 3 NaCl
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Does someone else here find the following data dubious?
"Compound 1 was characterized spectroscopically and
thermally. The material begins to decompose at 141°C with a
decomposition energy release of 1818 J/g."
I think they made a mistake on the units and that 1818 J/g (or kJ/kg) should rather be 1818 cal/g (or kcal/kg).
[Edited on 12-11-2012 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
A new article, for those interested in triazoles:
Nitrogen-Rich Bis-1,2,4-triazoles—A Comparative Study of Structural and Energetic Properties
Alexander A. Dippold, Thomas M. Klapötke
Chemistry - A European Journal 2012, early view (page numbers not yet assigned)
DOI: 10.1002/chem.201202483
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Davin
Harmless

Posts: 36
Registered: 5-12-2012
Member Is Offline
Mood: No Mood
|
|
Everyone knows about aminotetrazole and diaminotetrazole.
What about triaminotetrazolium? :-)
http://onlinelibrary.wiley.com/doi/10.1002/ejic.201200964/ab...
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Melam and Melamine could make interesting explosive salts. They are nitrogen-rich 
Rest In Pieces!
|
|
|
Solomon
Hazard to Self
 
Posts: 82
Registered: 24-6-2013
Location: Ancient Mines
Member Is Offline
Mood: FOR SCIENCE!
|
|
URL is a void link. But to all other scientists on this forum - I agree... we must begin searching for a method of synthesizing this material. If
anyone figures out a way to do it then call (805) 279-8556 and post your results.
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Solomon  | | URL is a void link. But to all other scientists on this forum - I agree... we must begin searching for a method of synthesizing this material. If
anyone figures out a way to do it then call (805) 279-8556 and post your results. |
Which link are you refering to?
|
|
|
SURT Tech.
Harmless

Posts: 7
Registered: 28-6-2013
Member Is Offline
Mood: No Mood
|
|
LOOKING FOR ADVICE. 4TH GENERATION POLYMER-BONDED THERMOBARIC EXPLOSIVE
Hello to all members of the forum. Considering the nature of our initiative it will be hard task to explain in details our work and goals in just a
single post, but we’ll try. The subject is somewhat touchy and we are more than sure, that we are the first ones who ever tried to do it in such a
“delicate” way.
Briefly. We are recently founded (private research) company from Bulgaria, engaged mostly in the field of blast enchased explosives. On 30.04.2013, on
the testing ground of one of the largest weapon producer in Bulgaria, we made the final tests of our thermobaric compositions (TBX – 2) with the
best hardware available in order to extract the maximum data needed to prove our conception. The results from the comparative tests (5 kg. charges
with pure A – 9 and standard mash type TB – RDX, IPN, Al) far exceeded our expectations and that’s the main reason for our desire to move it
abroad.
As well educated people who have access to the latest research papers and scientific publications, we have closely monitored, studied and tested every
promising thermobaric composition or design from the last decade. Based on this our composition is considered to be 4th generation thermobaric, which
(as we found) is capable to detonate and burn completely the metal fuel even in 50 gr. quantities. We also want to point that all expenses around the
research stages and the tests were paid by the name of our company (SURT Technologies) and considering this and the fact that we are private
researchers (and we are not engage with any government structures), we are completely free to sell our products abroad. The big question is how and is
this possible at all? With other word is it possible for private company abroad to sell the patent rights for the product or the license for the
manufacture to other country?
What are the main advantages of our composition?
1. Cutting edge solid state concept, analogous to the one used in the “Hellfire II AGM-114N”, but completed with the use of much cheaper, safer
and ready available materials that release even higher explosive power (16 MJ/kg or 4 times TNT equivalent for the energy of aerobic detonation, Pcj
25-30 GPa for the anaerobic detonation).
2. Lack of any volatile liquid monopropellants (like IPN in the Russian “Shmel” for example), which eases the production, handling and most
important - improves the storage stability. Not need of airtight or reinforced containers.
3. Energetically burning polymer binder, which achieves an effect similar to that of additionally introduced metal fuel into the composition. The
polymer also acts as catalyst to ensure the complete burning of the metal fuel into the atmosphere. The very first tests were conducted at a
temperature of -10 (minus ten) degrees Celsius, which again confirms the high versatility and the excellent performance of our thermobaric
compositions. It’s very important to note that our thermobaric compositions detonate completely even in 50 – 100 grams quantities, which is
impossible for any other polymer bonded thermobaric explosives. The widely used PBX compositions like PBXH-135 and AFX-757 give satisfactory results
only in large amounts, but in quantities less than 10 kilos they suffer from incomplete combustion of the metal fuel, which make them useless in small
calibers.
4. Inhibition. The coated with polymer metal particles and oxygen rich salts are protected from external influence during handling and storage, which
significantly extends the shelf life and stability of the produced ammunition.
5. Low sensitive to mechanical and thermal impact elements, which in turn puts these products in the category of so-called "insensitive" munitions.
Exceptionally high thermal stability, which makes them excellent filler for various supersonic munitions. The composition is stable for short time
heating up to 250 °C and its flash point is above 300 °C. The used polymer maintains its mechanical properties from -50 to more than 200 °C.
6. Inert. High resistance to oxidation even at high temperatures without the use of any anti-oxidants or other preserving agents. Zero hygroscopicity
and high humidity resistance of the final product. Samples of the described compositions have been stored so far for more than a year (from -15 to +45
°C) without any chemical or mechanical changes to the structure and the properties of the material.
7. Low production cost at about 10-15 USD per kilogram! All ingredients are high-tonnage products of the chemical industry. Our binding system
completely eliminate the problems associated with the classical "slurry" method such as high cost, low yield, the use of specialized equipment and
most importantly - the oxidation of the used metal fuels. That process can be done even "manually" or with the help of ordinary mechanical mixers.
Тhe resulting plastic / semi plastic mass can be charged into munitions using exactly the same methods as the ones used for the composite rocket
propellants. This makes the production cycle much simpler and eliminates the need of specialized equipment or the construction of separate production
lines. Factors, which shrink the production costs to minimum.
8. High charge density (more than 2 g/cc) in comparison with compositions based on polymers such as hydroxyl-terminated polybutadiene (HTPB). Very
good mechanical properties. Easy molding and pressing of the resulting thermobaric composition. Consistency of a solid rubber in polymerized state and
a paste in unpolymerized state, which can be extruded even through 1.5 mm opening.
9. Classification and environmental issues. Considering the legal issues around thermobaric weapons in recent years, our explosives can be classified
either as “thermobaric” or “enhanced blast”, because they have all the positive characteristics of the classic aluminized explosives (solid
state, low sensitivity similar to Tritonal, AFX-757, etc.), but with 2 - 4 times higher blast/pressure capabilities. These new explosives do not
contain any toxic products or evolves products (after detonation) with higher toxicity than Tritonal, AFX-757 or PBXH-135.
Furthermore. Right now we worked on another thermobaric composition based on completely new technology concept. Also advanced thermite charges and
grenades. Flash bang grenades with improved performance and cheap, non-toxic ingredients (lack of any heavy metal salts, etc.). We are also developing
Novel Software for computer simulations of different thermobaric compositions.
Well, that’s it generally. As we stated above, the subject is more than unconventional, but we are well educated, handle freely English and Russian
and have the huge willing to continue our development in the field.
We want to apologies in advance if we violate any rules of the forum or the post is made in the wrong thread.
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
My friend from the above post could not attach this picture. By the way, I helped him with the theoretical predictions before they did any tests...
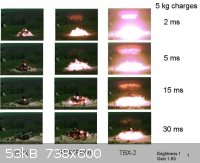
When logic and proportion have fallen sloppy dead...
|
|
|
Solomon
Hazard to Self
 
Posts: 82
Registered: 24-6-2013
Location: Ancient Mines
Member Is Offline
Mood: FOR SCIENCE!
|
|
I would love to know how you guys at SURT technologies synthesized TBX-2. I realize that you wouldn't want idea theft but I wouldn't do that. If you
give me this idea I will give you one of mine about a hydrogen computer concept  . Not that my idea is too practical, but you might be able to take it somewhere. What is the detonation velocity of TBX-2? . Not that my idea is too practical, but you might be able to take it somewhere. What is the detonation velocity of TBX-2?
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
Oh, it is just a thermobaric composition, not much synthesis is involved. The advantage is that it burns all fuel into the positive pressure phase and
yields 3-4 times TNT blast equivalent for 15 dolars/kg. Ammerican compositions have lower yield even by using extremely exotic chemicals like fluorine
or other active polymers etc ... and price rises above 50-100 dolars/kg... SURT has patent on this anyway. If you have any connections with the
industry, help us and connects us with. As we made it but have no connections and of course we want it to have real use...
Here is a short presentation, if you have problems downloading it tell me:
http://dox.bg/files/dw?a=df6fdacb82
Barely forgot, detonation velocity about 7500-8000 m/s.
[Edited on 28-6-2013 by simply RED]
When logic and proportion have fallen sloppy dead...
|
|
|
Solomon
Hazard to Self
 
Posts: 82
Registered: 24-6-2013
Location: Ancient Mines
Member Is Offline
Mood: FOR SCIENCE!
|
|
Thank you. Could I get the patent number detailing the creation of TBX-2 and the ratios of RDX to TBX-2. Just ask me if you want the idea for the
hydrogen computer. Are you the CEO of SURT technologies?
[Edited on 28-6-2013 by Solomon]
[Edited on 28-6-2013 by Solomon]
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
I just performed all theoretical predictions before compositions were tested. Engineering enhanced blast/thermobaric/SFAE whatever you call it is
extremely difficult. Even using HMX in compositions HMX/Al/HTPB does not help as the energy of detonation gets only 2-3 MJ/kg - less than needed to
ignite and burn all Al. American researchers tried to solve this by using active and/or high density polymers like in PBXIH-18. This partially solves
the problem but the price of the final product gets too high (it is already too high with HMX). Another attempt to solve the problem was the anular
design employed in the Hellfire 2 warhead. Apart from the price problem, a very bad processibility problem arises as the used fluorine polymers tend
to be too brittle or generally lack mechanical qualities. No practical sheme for loading the mixture was developed. So Hellfire 2 warhead was the only
one completed by this design AFAIK. Russian researchers, countrary, tried to apply low tech solution - adding monopropellant like IPN in the mixture.
Same problems with the low energy appeared and as you can see from the pictures in the presentation - IPN mixtures are not even capable of igniting
most of the metal powder! In fact RDX/IPN/Al was one of the worst performing in our tests, much worse than all alluminized PBXs, maybe even worse than
AN/NM/Al... Another tried method to ignite "those freaking inert" metal particles was adding oxidizer directly into the mix forming RDX/AP/Al/Polymer
like material. Good idea, except for the fact that those oxidizers remain inert during the detonation propagation and still the metal particles do not
ignite well if the mass of the material is less than 50 kilos.
So, we solved all those problems and here is the TBX-2 composition using new principle to be able to fully burn the fuel no matter in open, closed
space, winther conditions, wind etc. Please no more spam, PM me or the colegue above for further questions. I do not even know if the forum was the
right place to post this. Anyway, it was the ONLY place. Jane military and others rejected us and we could not post it anywhere else... So, all test
are over and no more evaluation is needed, now we are looking for connections with the industry.
When logic and proportion have fallen sloppy dead...
|
|
|
DubaiAmateurRocketry
National Hazard
   
Posts: 841
Registered: 10-5-2013
Location: LA, CA, USA
Member Is Offline
Mood: In research
|
|
Quote: Originally posted by simply RED  | I just performed all theoretical predictions before compositions were tested. Engineering enhanced blast/thermobaric/SFAE whatever you call it is
extremely difficult. Even using HMX in compositions HMX/Al/HTPB does not help as the energy of detonation gets only 2-3 MJ/kg - less than needed to
ignite and burn all Al. American researchers tried to solve this by using active and/or high density polymers like in PBXIH-18. This partially solves
the problem but the price of the final product gets too high (it is already too high with HMX). Another attempt to solve the problem was the anular
design employed in the Hellfire 2 warhead. Apart from the price problem, a very bad processibility problem arises as the used fluorine polymers tend
to be too brittle or generally lack mechanical qualities. No practical sheme for loading the mixture was developed. So Hellfire 2 warhead was the only
one completed by this design AFAIK. Russian researchers, countrary, tried to apply low tech solution - adding monopropellant like IPN in the mixture.
Same problems with the low energy appeared and as you can see from the pictures in the presentation - IPN mixtures are not even capable of igniting
most of the metal powder! In fact RDX/IPN/Al was one of the worst performing in our tests, much worse than all alluminized PBXs, maybe even worse than
AN/NM/Al... Another tried method to ignite "those freaking inert" metal particles was adding oxidizer directly into the mix forming RDX/AP/Al/Polymer
like material. Good idea, except for the fact that those oxidizers remain inert during the detonation propagation and still the metal particles do not
ignite well if the mass of the material is less than 50 kilos.
So, we solved all those problems and here is the TBX-2 composition using new principle to be able to fully burn the fuel no matter in open, closed
space, winther conditions, wind etc. Please no more spam, PM me or the colegue above for further questions. I do not even know if the forum was the
right place to post this. Anyway, it was the ONLY place. Jane military and others rejected us and we could not post it anywhere else... So, all test
are over and no more evaluation is needed, now we are looking for connections with the industry. |
Explosives with polymers ? cool, i have a small idea to decrease any sensitive explosive to 0 reactivity.
|
|
|
Solomon
Hazard to Self
 
Posts: 82
Registered: 24-6-2013
Location: Ancient Mines
Member Is Offline
Mood: FOR SCIENCE!
|
|
Simply Red, as far as an industry connection, I do know someone... dr. Robert Cassar of earth shift products. I may be able to get him to work with
you. Although earth shift has different types of things that they work on, maybe they would consider you of interest. I will talk to Robert's advisor
and see if I can get his attention. If however you accept this offer, you must remember that he lives in Hawaii and if I can get his attention you
will almost certainly have to fly out to hawaii. I will try and work out the details now... tell me if you want to go with it. Remember, earth shift
products is a multi billion $ company, they will be able to take you places. This will help me as well because if you guys can impress him, he will
pay more attention to me. Looking forward to the possibility of working with you!
|
|
|
simply RED
Hazard to Others
  
Posts: 209
Registered: 18-8-2005
Location: noitacoL
Member Is Offline
Mood: booM
|
|
Please no more spam in the main topic, address everything regarding TBX-2 as a personal message to SURT Tech. Yes, we are looking for every possible
way of cooperation. Solomon, please send personal message to SURT Tech. We can sell the license for production according to the patent to every
company that has the valid documents for production and trade of energetic materials or is a LEGAL researcher of energetic materials. Of course we are
looking O-N-L-Y for such industry contacts! And please no more spam, PM.
[Edited on 29-6-2013 by simply RED]
[Edited on 29-6-2013 by simply RED]
When logic and proportion have fallen sloppy dead...
|
|
|
Solomon
Hazard to Self
 
Posts: 82
Registered: 24-6-2013
Location: Ancient Mines
Member Is Offline
Mood: FOR SCIENCE!
|
|
New novel energetic materials have been observed. N60 is a new energetic material with a detonation velocity of 17000 m/s  . I am reading a PDF here: http://www14.atpages.jp/~highenergy/hb_jx/File-hb-005-CESW20... . There is a chart comparing the promise between molecules composed only of
nitrogen atoms. The N60 molecule is the graphene of the energetic materials world. I watched the staircase implementation of the big bang theory and
in it Leonard is working on a new rocket fuel for the government with over 8,000,000 newtons of thrust. Leonard shows his friends the new fuel in his
apartment, however he fails to take into account that his rocket fuel was for a full scale rocket, and he mixes it in a tovex coated with Iridium and
exposes far too much of the propellant's surface area to the Iridium resulting in the accident seen on the big bang theory season 3 the staircase
implementation. Buy it on itunes and skip to around 17 minutes to see an awesome novel rocket fuel based on a real one developed in 2003. . I am reading a PDF here: http://www14.atpages.jp/~highenergy/hb_jx/File-hb-005-CESW20... . There is a chart comparing the promise between molecules composed only of
nitrogen atoms. The N60 molecule is the graphene of the energetic materials world. I watched the staircase implementation of the big bang theory and
in it Leonard is working on a new rocket fuel for the government with over 8,000,000 newtons of thrust. Leonard shows his friends the new fuel in his
apartment, however he fails to take into account that his rocket fuel was for a full scale rocket, and he mixes it in a tovex coated with Iridium and
exposes far too much of the propellant's surface area to the Iridium resulting in the accident seen on the big bang theory season 3 the staircase
implementation. Buy it on itunes and skip to around 17 minutes to see an awesome novel rocket fuel based on a real one developed in 2003.
|
|
|
Solomon
Hazard to Self
 
Posts: 82
Registered: 24-6-2013
Location: Ancient Mines
Member Is Offline
Mood: FOR SCIENCE!
|
|
Is there even a remote chance that anyone knows the detonation velocity of hydrazinium azide hydrazinate or will I have no choice but to synthesize it
and find out as I can't find that information anywhere. Sending out a call of desperation here.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
8100 m/s = HN3
3400 m/s = NH4N3
Hydrazine is detonable on it's own so the Azide is much more energetic than
Ammonium Azide. Guessing ∆Hf to be + 219 kJ/mol , and density of 1.8 g/cc
entering into Engager's Detonation Calculator Utility ( this link )
www.sciencemadness.org/talk/viewthread.php?tid=11195&pag...
obtains 7950 m/s for N2H4•HN3
According to this paper the vod of N2H4•HN3 is comparable to that of RDX at
the same density.
www.chemie.uni-muenchen.de/ac/klapoetke/content/research/pos...
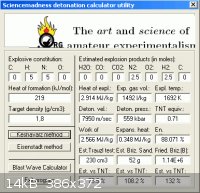
The Hydrazine adduct will have lower overall energy so the vod must be
substantially less. The density is given as ρ=1.32 g/cc here _
http://onlinelibrary.wiley.com/doi/10.1002/1521-4087(200110)...
Guessing ∆Hf to be + 154 kJ/mol and entering into Engager's Detonation Calculator Utility
obtains 5426 m/s for (N2H4)2•HN3
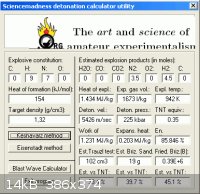
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
I do not think it actually is. Perhaps you mistyped?
Hydrazine has a substantial heat of formation, but it was my understanding that, because of the N-H hydrogen bonding, it is not enough to overcome the
energy it takes to gasify surrounding molecules, and thus decomposition can only be sustained by a catalyst.
I have seen the detonation velocity listed somewhere before for this compound. It was lower than that. In fact, I remember it being somewhat lower
than HN3, though it was still substantially higher than NH4N3. A measurement difference could possibly be due to the velocity being dependent on the
diameter of the charge, but you should also be aware that calculation algorithms often tend to overestimate the power of explosives that contain very
high proportions of nitrogen.
A quick look at the data plot from one source seems to suggest an observed detonation velocity of around 7.6-7.7 km/s for N2H4•HN3
(G. S. Yakovleva, R. Kh. Kurbangalina, L. N. Stesik, Fiz. Goreniya Vzryva 1974, 10(2), 270-274.)
[Edited on 28-7-2013 by AndersHoveland]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Hydrazine gas does propagate detonation see page 364 here _
Combustion and Detonation Characteristics of Hydrazine and Its Methyl Derivatives
www.sciencemadness.org/talk/viewthread.php?tid=874#pid254995
According to Encyclopedia of Explosives , Fedoroff , H-192
Liquid anhydrous hydrazine is nonexplosive /
completely insensitive to shock , friction or electric discharge.
• the vapor may be exploded without air
.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
On a similar line as what PHILOU Zrealone proposes here _
www.sciencemadness.org/talk/viewthread.php?tid=1970&page...
Tetrabromoethylene with Lithium Azide should yield an aromatically stable
Br2C=CBr2 + 4 LiN3 => 4 LiBr + (N3)2C=C(N3)2 / / => N6C=CN6
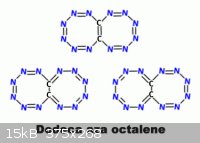
From what I understand his type direct substitution generally only applies to carbon
single bonded to another carbon , a primary alkyl. Secondary alkyl , those carbons
bonded to two other carbons are much less active for this type reaction. Tertiary
alkyl carbons are not susceptible at all. It is also the case with alkenes , carbon
with a double bond to another carbon. Alkenes are instead susceptible to breaking
a bond to add more atoms or groups. Aryl ( aromatic benzene ring ) carbons have
been demonstrated as alkyl carbons to be labile to substitution induced by catalytic
action of for example copper.
We know diatomic molecular Bromine and Lithium azide react violently on contact.
Bromine substitutes the azide and the bromyl azide formed dissociates. It seems
that minor activation energy would be required to induce a metathetical exchange
with Tetrabromoethylene a Colorless crystal that Melts at 55 ~ 56 °C ,
Boiling point 226 to 227 °C Insoluble in water, soluble in alcohol , ether and other
organic solvents. Bond dissociation energies indicate the reaction to be exothermic.
Approximate values are , C-Br = 80 kcal/mol , LiBr = 100 kcal/mol , LiN3 = 50 kcal/mol
C-N3 = < 80 kcal/mol
.
[Edited on 11-8-2013 by franklyn]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
A new article that might be of interest:
Energetic N-Trinitroethyl-Substituted Mono-, Di-, and Triaminotetrazoles
Qinghua Zhang, Jiaheng Zhang, Damon A. Parrish, Jean'ne M. Shreeve
Chemistry - A European Journal, 19(33), 2013, 11000–11006.
DOI: 10.1002/chem.201300994
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by franklyn  | On a similar line as what PHILOU Zrealone proposes here _
www.sciencemadness.org/talk/viewthread.php?tid=1970&page...
Tetrabromoethylene with Lithium Azide should yield an aromatically stable
Br2C=CBr2 + 4 LiN3 => 4 LiBr + (N3)2C=C(N3)2 / / => N6C=CN6
From what I understand his type direct substitution generally only applies to carbon
single bonded to another carbon , a primary alkyl. Secondary alkyl , those carbons
bonded to two other carbons are much less active for this type reaction. Tertiary
alkyl carbons are not susceptible at all. It is also the case with alkenes , carbon
with a double bond to another carbon. Alkenes are instead susceptible to breaking
a bond to add more atoms or groups. Aryl ( aromatic benzene ring ) carbons have
been demonstrated as alkyl carbons to be labile to substitution induced by catalytic
action of for example copper.
We know diatomic molecular Bromine and Lithium azide react violently on contact.
Bromine substitutes the azide and the bromyl azide formed dissociates. It seems
that minor activation energy would be required to induce a metathetical exchange
with Tetrabromoethylene a Colorless crystal that Melts at 55 ~ 56 °C ,
Boiling point 226 to 227 °C Insoluble in water, soluble in alcohol , ether and other
organic solvents. Bond dissociation energies indicate the reaction to be exothermic.
Approximate values are , C-Br = 80 kcal/mol , LiBr = 100 kcal/mol , LiN3 = 50 kcal/mol
C-N3 = < 80 kcal/mol
.
[Edited on 11-8-2013 by franklyn] |
What I exposed was N4C2N4 with a naphtalene structure or N4C=CN4 with a bistetrazolene structure.
Both are fully aromatic and this means each N-N or N=N is nearly equivalent in bond lenght and in the aromatic plane of the molecule. The aromatic
resonance has a stabilizing effect as seen with tetrazoles (heteroaromatic pentaring).
In your N6C2N6 molecule the octacycles are no more really aromatic (bended out of the plane and twisted) and some of the N-N or N=N becomes
unequivalent what is the weakest link... This is very detrimental to the stability of high nitrogen content molecules with >3 polynitrogen atom
chains.
So a site with -N=N- will more likely expell N2 (N#N) generating a lot of energy probably sufficient to trigger the detonation of the rest of the
product...if the molecule survives the decomposition, there would be a chance it rearranges into the putative stabler molecule I thought about.
N6C2N6 --> N4C2N4 + 2 N2
Or the molecule will simply remain uncyclized as tetraazidoethylene. Tetraazidoethen would be (like tetracyanoethene or tetranitroethene) a good
scavenger of electrons...electron charge transfer and it can be trapped by dienic structures CH2=CH-CH=CH2 (Diels-Alder reactions).
If tetrahaloethene is not reactive enough for the substitution, alternatively tetrahalo-1,2-dinitroethane will be...leading to a sensitive perfect OB
HE...
O2N-CH2-CH2-NO2 --Br2 or Cl2/mild base--> O2N-CBr2-CBr2-NO2 or O2N-CCl2-CCl2-NO2
By vitue of the close relationship to chloropicrine, those nitro-halogenated compounds must be considered highly toxic agents.Their halogen are
labiles because of the nitro group vicinity.
O2N-CX2-CX2-NO2 --NaN3/solvent--> O2N-C(N3)2-C(N3)2-NO2 + 4 NaX
Those polyazido-nitro compounds will probably display the same kind of hydrolysability than their halogen cousins leading to toxic HN3 gassing and be
hell friction/shock sensitive. Azido group being a pseudohalogen...
[Edited on 27-8-2013 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Davin
Harmless

Posts: 36
Registered: 5-12-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by franklyn  | On a similar line as what PHILOU Zrealone proposes here _
www.sciencemadness.org/talk/viewthread.php?tid=1970&page...
Tetrabromoethylene with Lithium Azide should yield an aromatically stable
Br2C=CBr2 + 4 LiN3 => 4 LiBr + (N3)2C=C(N3)2 / / => N6C=CN6
From what I understand his type direct substitution generally only applies to carbon
single bonded to another carbon , a primary alkyl. Secondary alkyl , those carbons
bonded to two other carbons are much less active for this type reaction. Tertiary
alkyl carbons are not susceptible at all. It is also the case with alkenes , carbon
with a double bond to another carbon. Alkenes are instead susceptible to breaking
a bond to add more atoms or groups. Aryl ( aromatic benzene ring ) carbons have
been demonstrated as alkyl carbons to be labile to substitution induced by catalytic
action of for example copper.
We know diatomic molecular Bromine and Lithium azide react violently on contact.
Bromine substitutes the azide and the bromyl azide formed dissociates. It seems
that minor activation energy would be required to induce a metathetical exchange
with Tetrabromoethylene a Colorless crystal that Melts at 55 ~ 56 °C ,
Boiling point 226 to 227 °C Insoluble in water, soluble in alcohol , ether and other
organic solvents. Bond dissociation energies indicate the reaction to be exothermic.
Approximate values are , C-Br = 80 kcal/mol , LiBr = 100 kcal/mol , LiN3 = 50 kcal/mol
C-N3 = < 80 kcal/mol
.
|
Franklyn: Take a look at the introduction of my article here (http://pubs.acs.org/doi/abs/10.1021/ja310384y?journalCode=ja...) for a quick overview of why such a system is unlikely to be stable and for
general considerations in the design of long-chained nitrogen species.
The electron-rich nature of such a ring allows the donation of electron density from the N-lone pairs into the N-N sigma antibonding orbitals,
destabilizing the ring to the point of (likely) non-existence. This is why unsubstituted 1,2,3,4-tetrazines are so rare (1 example lit). However the
key to long-chain nitrogen systems is the removal of LP electron density on alternating N atoms within the chain, increasing the energy difference
between these orbitals and stabilizing the system. This is why 1,2,3,4-tetrazine-1,3-dioxides are so much more stable.
Your species is likely analogous, so in the event that 3 oxides can be introduced to each ring, it may be more stable. It would also then have a
positive OB.
|
|
|
| Pages:
1
..
6
7
8
9
10
..
23 |