| Pages:
1
..
6
7
8
9
10
..
40 |
strontiumred
Harmless

Posts: 36
Registered: 6-7-2007
Location: Southern England
Member Is Offline
Mood: Delocalised
|
|
Here is some blue sodium hypomanganate, Na3MnO4.
Thanks to Mixell for the method.
12NaOH + 4MnO2 + O2 ---> 4Na3MnO4 + 6H2O.
<a href="http://www.flickr.com/photos/strontiumred/5894390500/" title="Hypomanganate.jpg by strontiumred, on Flickr"><img
src="http://farm6.static.flickr.com/5151/5894390500_c959d89115.jpg" width="450" height="434" alt="Hypomanganate.jpg"></a>
[Edited on 2-7-2011 by strontiumred]
[Edited on 2-7-2011 by strontiumred]
[Edited on 2-7-2011 by strontiumred]
|
|
|
spotlightman1234
Harmless

Posts: 17
Registered: 16-4-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by strontiumred  | Here is some blue sodium hypomanganate, Na3MnO4.
Thanks to Mixell for the method.
12NaOH + 4MnO2 + O2 ---> 4Na3MnO4 + 6H2O.
<a href="http://www.flickr.com/photos/strontiumred/5894390500/" title="Hypomanganate.jpg by strontiumred, on Flickr"><img
src="http://farm6.static.flickr.com/5151/5894390500_c959d89115.jpg" width="450" height="434" alt="Hypomanganate.jpg"></a>
[Edited on 2-7-2011 by strontiumred]
[Edited on 2-7-2011 by strontiumred]
[Edited on 2-7-2011 by strontiumred] |
Wow, what a beautiful compound! could you please send a link to the
thread with the synthesis, or tell me how to make it?
|
|
|
Xenomorph
Harmless

Posts: 17
Registered: 23-4-2011
Member Is Offline
Mood: No Mood
|
|
Here is some nitroguanidine I made. First one is impure stuff, second one is recrystalized from boilig water. I will use it to make aminoguanidine,
but I am quite busy right now. Is it safe to store this in dry form? I have read this is pretty insensitive and also I have read this is stored with
20% water not in dry form.
   
|
|
|
ItalianChemist
Hazard to Others
  
Posts: 172
Registered: 26-1-2011
Location: Italy
Member Is Offline
Mood: No Mood
|
|
this is my sodium hypomanganate(V), obtained from sodium hydroxide and MnO2

|
|
|
strontiumred
Harmless

Posts: 36
Registered: 6-7-2007
Location: Southern England
Member Is Offline
Mood: Delocalised
|
|
Hi,
Mixell suggested this method:
| Quote: |
Mix (very well) molar amounts ( 3:1 ratio) of NaOH (prills or powder) with a finely divided manganese dioxide powder, I used 10-15 grams of NaOH. Put
it all in a beaker and mix well. Put it on a hotplate and heat the hotplate to about 300 degrees, so if you put a prill of NaOH directly on the
hotplate- it will melt (but do not heat above that). Steer and divide possibly accumulating masses of matter every few minutes. Check every 10 min a
small sample of the mix, when all of it will be blue (allow for a tiny bit of unreacted MO2 to remain, if they refuse to react, you can't win them
all) switch off the hotplate and allow it to cool while stirring every 2-3 minutes, thats basically it. Be prepared for the fact that the bottom of
the beaker will be slightly damaged. If all goes well- post some pictures, May be I will do the same in a few days.
|
I don't own a hotplate (yet) so I simply ground 350mg manganese dioxide and 550mg of sodium hydroxide together and heated over an alcohol lamp for
about 15 seconds, removed for 5 seconds and continued like this for about 20 minutes or so. Obviously any glass will be attacked by the hydroxide so
if you have a steel crucible that would produce better results.
One thing is you have to keep the solid totally dry. Any water formed must not be allowed to consense back into the mix as it will cause the
following reaction. 2Na3MnO4 + 2H2O ---> Na2MnO4 +MnO2 + 4NaOH.
DerAlte has posted some great info in the permanganates thread including this:
MnOxy.doc
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
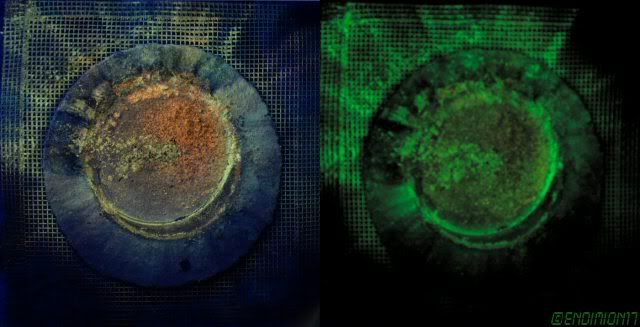
Some Oxide spots on Nb by placing drops of KCl solution on a Nb sheet and connecting the sheet to + and putting a resistor end into the blob of KCl
solution. (sheet an Anode, resistor end the Cathode).
The different colours are different thicknesses of Oxide. Better pictures on web if you look.
Dann2

[Edited on 8-8-2011 by dann2]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|

Amethyst coloured Ferric alum ‘geode’ (about 600 g of ammonium ferric sulphate dodecahydrate)
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Wow, a pretty photo thread.
Here's my contribution:
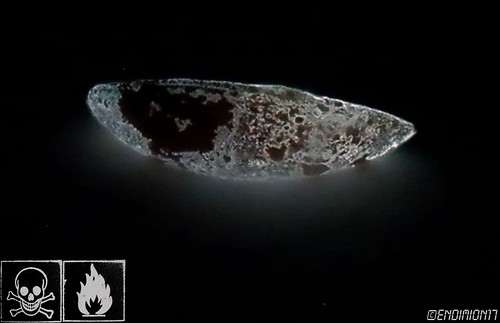
WP I photographed few days ago.
ZnS phosphorescent dust.

Fresh iron sulfate heptahydrate.
some rupert drops against a polarizer
my first handmade liebig cooler - never actually tried how it works...
my hand grounding a germicidal tube - It was turned off, but connected to the mains 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Love that zinc sulphide!

About 500 g (about 100 mm wide) of ferrous sulphate heptahydrate, recrystallised from garden grade (unknown mixture of hydrates with an anti-caking
agent - bweurk!)
[Edited on 23-8-2011 by blogfast25]
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
@Endimion17:
That FeSO4 looks identical to the masses of crystals I'm getting from my current project of dissolving Nd magnets in sulfuric acid, neat! Your WP is
beautiful too.
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
Large quartz crystals, lab made  One was traded to Theo Gray. One was traded to Theo Gray.

I can post more crystals of salts and stuff, I completely forgot I had a box of sealed samples. Also stuff from my friend Ivan at www.periodictable.ru 
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Not aesthetically pleasing but pretty, in the sense that I was able to observe and photo the fleeting existence of this complex.

Triiron Dodecacarbonyl - Fe3(CO)12
Tank
|
|
|
bob800
Hazard to Others
  
Posts: 240
Registered: 28-7-2010
Member Is Offline
Mood: No Mood
|
|
That's a really neat homemade condenser! Did you use an ordinary bunsen burner or blowtorch to melt the glass? I've been trying to make a crude Allihn
condenser, but I can never get the bulbs to form... maybe my propane bunsen isn't hot enough.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Curious as to how you made that complex!
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 860
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
Benzoic acid crystals made from sodium benzoate and NaCl. Maybe some of you have seen it in the Wikipedia article..? 

This just in: 95,5 % of the world population lives outside the USA
Please drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
Endimion17
International Hazard
    
Posts: 1468
Registered: 17-7-2011
Location: shores of a solar sea
Member Is Offline
Mood: speeding through time at the rate of 1 second per second
|
|
Quote: Originally posted by bob800  | | That's a really neat homemade condenser! Did you use an ordinary bunsen burner or blowtorch to melt the glass? I've been trying to make a crude Allihn
condenser, but I can never get the bulbs to form... maybe my propane bunsen isn't hot enough. |
Thanks. Propane-butane blowtorch, no additional oxygen. Bunsen can't do it because flamerorking requires sharp flames. It's ok for sooty annealing,
though.
It's very difficult to make because of several glass connections and lots of stresses, but now I understand the price of a professional cooler.
Your bunsen burner probably is hot enough. The problem is in heat delivery i.e. gas consumption. Some burners consume less gas, so their energy output
is lower, though the temperature is sufficient.
@Wizzard: lab made? w00t!
[Edited on 24-8-2011 by Endimion17]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
How do you make them?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Isn't quartz supposed to be somewhat soluble in super saturated steam or something like that?
Otherwise, crystalisation from a melt?
Good luck with both in a home lab!
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
Oh, I didn't make them  Somebody with a large autoclave did, and these were
produced as the process was being learned, judging by the shapes. Somebody with a large autoclave did, and these were
produced as the process was being learned, judging by the shapes.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
That must be a really small $20 bill
|
|
|
sternman318
Hazard to Others
  
Posts: 121
Registered: 21-4-2011
Member Is Offline
Mood: No Mood
|
|
Ditto!
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
In my case, it's the direct union of hot CO with hot, finely divided iron. Some of the pentacarbonyl converts to the [solid] dodecarbonyl which
collects on and around the vent cracks.
Tank
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
You might find this pretty, or not.
This is an aluminium (air) cell, an exotic cell that not many people are familiar with. The anode is made of aluminium foil and the cathode is made of
graphite rods from pencils. The extra twist is that I'm using Fenton's reagent as an oxidiser instead of air, which allows a much higher power output,
as shown.
The white LED is hooked up to a joule thief because it needs 3V to operate while the cell only supplies half of that.
In my opinion, this surely beats a lemon battery 
It doesn't show up very well on the camera, but the white LED is bright enough to give retina burns if you stare into it for a few seconds.

If you would like a sense of scale, the glass jar is about 10cm tall (4 inches).
[Edited on 9-2-2011 by White Yeti]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Nice! You could put 2 cells in series to get enough voltage. What's a joule thief?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
I could have put two cells together to get enough voltage, but I was running out of chemicals at the time (I didn't even have any iron acetate
catalyst!), I barely had enough to build one cell.
I'm restocking in peroxide tomorrow, I have some fresh iron salts I prepared this morning to play with, and I have some new pencils and aluminium
foil.
A joule thief is a small electronic circuit used to boost voltage from a low voltage source. If you try to light a white LED with a fully charged AA
battery, you will find out that it won't light up because it needs at least 3V in order to function. Lithium button cells have voltages exceeding 3V
so they can light up white LEDs on their own, but all the other cells, including this one, cannot.
|
|
|
| Pages:
1
..
6
7
8
9
10
..
40 |