| Pages:
1
..
68
69
70
71
72
..
81 |
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
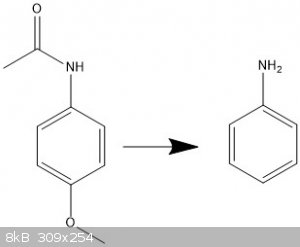
Does anyone know a viable way to remove the methoxy and acetyl groups without having to reattach the amino group?
I really don't know where to go here and any help would be hugely appreciated.
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
What type of propellant do you think is being used? The brown smoke in the beginning I believe is from metal oxides.... but the thick white smoke
seems peculiar... ?
Any chance they are using a EM we are not familiar with in the west.... my understanding is the Russians are ahead in explosive science.
https://www.foxnews.com/world/see-it-russia-test-launches-ne...
And here is a better video.
https://m.youtube.com/watch?v=qLV1Ao9QkpE
[Edited on 7-6-2019 by MineMan]
|
|
|
maldi-tof
Harmless

Posts: 39
Registered: 3-4-2019
Member Is Offline
|
|
Quote: Originally posted by Simoski  | Are any copper salts fat soluble?
May not seem like an energetic material type of question, but if there are any I will disolve it is lard or tallow, add potassium chlorate and have me
a new blue / green pyrotechnic star mix
[Edited on 23-5-2019 by Simoski] |
Should organocopper compounds do the trick?
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I have noticed that some old primary charges in detonators etc contain lead thiocyanate. I have made a little of this compound and it is not the least
bit energetic so what is its purpose in these compositions? An exotic fuel?
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
From wikipedia
"Lead(II) thiocyanate is a compound, more precisely a salt, with the formula Pb(SCN)2.It is a white crystalline solid, but will turn yellow upon
exposure to light. It has use in small explosives, matches, and dyeing. Like other metal cyanides, Lead thiocyanate explodes on heating when
mixed with sodium nitrite. Lead thiocyanate is used in explosives, specifically an ingredient in primers for small-arms cartridges, safety
matches, and to reverse aniline black dyeing (Gideon)."
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
I just found few AAAA Batteries from few 9v Batteries. I want to use their cases for caps, anyone have any idea how to remove everything and clean
them inside ? It looks quite tricky.
|
|
|
James Ikanov
Hazard to Self
 
Posts: 81
Registered: 12-7-2015
Location: Alaska
Member Is Offline
Mood: Zen
|
|
Quote: Originally posted by underground  | | I just found few AAAA Batteries from few 9v Batteries. I want to use their cases for caps, anyone have any idea how to remove everything and clean
them inside ? It looks quite tricky. |
What kind of 9V battery? Do the AAAA's have tabs welded together between them?
“To do good work one must eat well, be well housed, have one's fling from time to time, smoke one's pipe, and drink one's coffee in peace” -
Vincent Van Gogh
|
|
|
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
I have been reading about chlorate based explosives in Chemistry of Powder and Explosives by Tenney Davis. I noticed that sodium chlorate based
compositions were said to be slower and less powerful than their potassium counterparts. That is inspite of sodium chlorate having similar heat of
decomposition to potassium chlorate, and 11 or 12% more oxygen per unit mass.
Were the sodium chlorate mixtures damp, or is their some other factor I am not taking into account here?
Put that in your pipe and smoke it!
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Sometimes energetics materials behave strangely, despite assumptions based on their basic properties. Nothing humidity of NaClO3. Similarly as ice
which is lighter than water. And only attempt and watching showes the truth... ...LL ...LL
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by James Ikanov  | Quote: Originally posted by underground  | | I just found few AAAA Batteries from few 9v Batteries. I want to use their cases for caps, anyone have any idea how to remove everything and clean
them inside ? It looks quite tricky. |
What kind of 9V battery? Do the AAAA's have tabs welded together between them? |
Here is an example

[Edited on 7-8-2019 by underground]
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
Can i make basic cobalt carbonaten from the nnitrate? I cant Find a supplier..
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
It seems likely that the basic carbonate has a rather low solubility and that the nitrate has a high solubility. If that is the case it is probably
quite easy to do a simple metathesis, although you may need to adjust pH.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
I currently have a disagreement with people working in the oil industry but I trust Sciencemadness a bit more on this. Specifically since some of you
have done this kind of fractionnal distillation.
Is there or is there not benzene (C6H6, I know there is toluene, xylenes and others) in diesel fuel ?
Specifically diesel fuel. I know it's present in crude and in regular gasoline. Any details will be welcome.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
markx
National Hazard
   
Posts: 646
Registered: 7-8-2003
Location: Northern kingdom
Member Is Offline
Mood: Very Jolly
|
|
As far as I can perceive the continuous fractionation process used for crude oil separation, there is in principle no possibility or completely
removing components via this method or getting a very delicate separation. And it is not really needed for the end purpose of the oil industry.
The feed is entering the tower at about middle height and then proceeds to reditribute itself on the plates under reflux according to boiling points
of the fractions. Inexorably the components of the mix have to move through the plates in the column continuously since the feed is entering the
column continuously. Thus the fractions taken off from the various plates are always somewhat contaminated with the components that are still
migrating through the column to "find their place in the system".
On the other hand a batch fractionation has no constant infeed and therefore one can deplete the boiler contents one by one according to rising
boiling points as far the separation of the column allows for it. Thus a much finer separation of components can be achieved.
But of course the continuous fractionation of crude oil is not the only method that is used in oil refining and I'm sure it is possible to separate
benzene from diesel fuel by other means if needed to do so.
Exact science is a figment of imagination.......
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Hydrogen peroxide / urea
Has anyone any expirience with ?
https://en.m.wikipedia.org/wiki/Hydrogen_peroxide_-_urea
It is not a primary but can be detonated. I have no idea for its properties, sensitivity VoD e.t.c. It may be a good way to use some peroxide.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
No experience, but I was interested when I first learned of this too.
From the fact you can ship an lb. of it without HazMat fees in the US mail, I would guess it's pretty insensitive...
https://www.firefox-fx.com/ChemU-Z.htm
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Yea, it is actually a mixture of urea and 100% HP by 1:1 Not even a new material. I guess it would be at the range of +5000m/sec VoD and seems like a
good way to use some HP. Most of the peroxides are super sensitive but UHP is not.
[Edited on 12-11-2019 by underground]
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by markx  | As far as I can perceive the continuous fractionation process used for crude oil separation, there is in principle no possibility or completely
removing components via this method or getting a very delicate separation. And it is not really needed for the end purpose of the oil industry.
The feed is entering the tower at about middle height and then proceeds to reditribute itself on the plates under reflux according to boiling points
of the fractions. Inexorably the components of the mix have to move through the plates in the column continuously since the feed is entering the
column continuously. Thus the fractions taken off from the various plates are always somewhat contaminated with the components that are still
migrating through the column to "find their place in the system".
On the other hand a batch fractionation has no constant infeed and therefore one can deplete the boiler contents one by one according to rising
boiling points as far the separation of the column allows for it. Thus a much finer separation of components can be achieved.
But of course the continuous fractionation of crude oil is not the only method that is used in oil refining and I'm sure it is possible to separate
benzene from diesel fuel by other means if needed to do so.
|
Thanks Marks.
That answers and goes beyond my question.
In the meantime I found this very interesting paper:
https://www.researchgate.net/publication/288133039_The_compo...
Quite interesting to compare the results of what goes in the tanks with what comes out of the exhaust.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Hydrogen Peroxide Urea can haz detonations?
On a quick look, someone thinks this is a "sleeper", awaiting the first major industrial accident where regulators are forced to notice that yes,
indeed, it can go "boom"- As was ammonium nitrate before the Oppau disaster and nitro methane before the two 1958 Niagara NY and Pulaski IL railroad
tank car explosion disasters.
https://onlinelibrary.wiley.com/doi/abs/10.1002/prep.2016001...
| Quote: |
It is not sensitive to impact and friction. However, we demonstrated that UHP (ρ=0.93 g cm−3; packed into a steel pipe with inner diameter of
206 mm) detonates with experimental velocity of detonation (VOD) of 3780 m s−1. Moreover, for UHP with maximal theoretical density
(ρ=1.43 g cm−3), the calculated VOD reaches 5219 m s−1. Based on our findings, we recommend that present regulations regarding the
handling, storage and transportation of the UHP should be revised, especially in cases, where UHP is kept on a large scale, under confinement and at
places where the temperature can reach above 60 °C.
|
From other information on industrial uses, I suspect that a sample of this contaminated with a suitable decomposition catalyst (Sodium tungstate?)
might be more inclined towards "friskiness".
[Edited on 11-12-2019 by Bert]
[Edited on 11-12-2019 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bert  | On a quick look, someone thinks this is a "sleeper", awaiting the first major industrial accident where regulators are forced to notice that yes,
indeed, it can go "boom"- As was ammonium nitrate before the Oppau disaster and nitro methane before the two 1958 Niagara NY and Pulaski IL railroad
tank car explosion disasters.
https://onlinelibrary.wiley.com/doi/abs/10.1002/prep.2016001...
| Quote: |
It is not sensitive to impact and friction. However, we demonstrated that UHP (ρ=0.93 g cm−3; packed into a steel pipe with inner diameter of
206 mm) detonates with experimental velocity of detonation (VOD) of 3780 m s−1. Moreover, for UHP with maximal theoretical density
(ρ=1.43 g cm−3), the calculated VOD reaches 5219 m s−1. Based on our findings, we recommend that present regulations regarding the
handling, storage and transportation of the UHP should be revised, especially in cases, where UHP is kept on a large scale, under confinement and at
places where the temperature can reach above 60 °C.
|
From other information on industrial uses, I suspect that a sample of this contaminated with a suitable decomposition catalyst (Sodium tungstate?)
might be more inclined towards "friskiness".
[Edited on 11-12-2019 by Bert]
[Edited on 11-12-2019 by Bert] |
Yes, Bert thus has been on my radar too... was actually hoping it wouldn’t pop up on the form because it is essentially a insensitive secondary that
can be shipped without any ATF or hazmat.... afraid even posting on here can change that. I thought it would have good potential for a binary... such
as mixed with NM. 5200m/s isn’t too bad. And it’s cheap too! So wish thus was still my secret, but cats out of the bag!
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Nitromethane is a tad low on Oxygen. The urea peroxide is too.
How 'bout trying it in a mixture with an oxidizer or an oxygen positive HE.
In very small quantities, mind you!
My first thought was how it might behave towards admixture of a nice thin NC/NG jelly. My second thought was, I like my hands too much to find out.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Microtek
National Hazard
   
Posts: 869
Registered: 23-9-2002
Member Is Offline
Mood: No Mood
|
|
I experimented with urea peroxide many years ago. The synthesis was easy and high yielding, and the product was surprisingly insensitive. It burned in
a manner that made me think "propellant" so I experimented a little with it as an alternative to NC.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by Microtek  | | It burned in a manner that made me think "propellant" so I experimented a little with it as an alternative to NC. |
Did you get it to perform in a rocket motor? The source I posted is, of course, a high power rocketry materials supplier.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Microtek  | | I experimented with urea peroxide many years ago. The synthesis was easy and high yielding, and the product was surprisingly insensitive. It burned in
a manner that made me think "propellant" so I experimented a little with it as an alternative to NC. |
Alternative to NC... so can it be used as a binder?
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
ETN vs PETN
I did some tests with both ETN and PETN and for some reason, ETN was always more powerfull-brisant than PETN. ETN got a few % of vaseline too. The
containers were exactly the same and i hand-pressed them as much as i could. I thought PETN was more powerfull but ETN just did more damage and
penetrate even further. It was a small scale test like 4g, maybe ETN is more bristant than PETN but PETN may be better in bigger quantities.
[Edited on 26-12-2019 by underground]
|
|
|
| Pages:
1
..
68
69
70
71
72
..
81 |