| Pages:
1
..
67
68
69
70
71
..
77 |
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Did you produce this yourself? If, how did you packed and sealed it?
Meanwhile, cleaning and drying a small batch of acetone.

[Edited on 22-9-2020 by Fyndium]
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
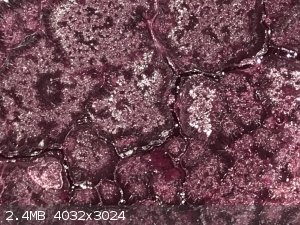
Cobalt formate crystals forming as the solution cools
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Rounded up eight old school mercury switches:

Yield of mercury:
 
I didn't have any mercury, so this was a cool find!
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
some Lead Iodide from yesterday
[Edited on 2-10-2020 by MidLifeChemist]

|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
These were sights for sore eyes to me... I'd been fighting a losing battle against tomato hornworms in my garden. The little monsters are devastating!
Things turned around when I learned that they're actually fluorescent under ultraviolet light. I can go out at night with my uv flashlight and just
pick them off the plants!
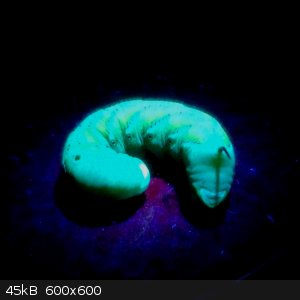
I don't kill all of them, though. I found a couple that were completely still and had these white things sticking out of their backs. It turns out
those have been parasitized by a specialist wasp. Since they're no longer chomping and they're hosting beneficial insect predators, these can stay

The more I looked into this wasp the weirder things got. Apparently it actually carries an endogenous virus in its genome, which it uses to infect the
caterpillar as part of the parasitic process. Most of the time a virus genome is compact to the point of heavy overprinting, but this virus contained
not only considerable noncoding sequence, but also introns! This, and a few other odd facts have led to the idea that it might actually be a new virus
in the process of emergence, rather than an ancient insertion. 
Attachment: Espagne et al. - 2004 - Genome sequence of a polydnavirus Insights into symbiotic virus evolution.pdf (189kB)
This file has been downloaded 405 times
It also turns out there is a second wasp which parasitizes the first, a situation called hyperparasitism. It's a hyperpest!!
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
I bought a bunch of small switches ("tilt" switches, maybe) some time ago, but I haven't decided how I want to break them. I suspect I'll get more
powdered glass than Hg!
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
I cracked them on one end, held down in a beaker, with a pair of pliers. The bits of glass that fell in with the mercury was no problem, as it doesn't
"wet". The mercury poured off clean.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
I find if you hold them by the wire connections, with the wire connections pointing down. Then use side cutters to take the to off. Then pour the
mercury into you breaker. I have opened many switched and this has always worked well.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Am I the only one who gets breaking bad vibes from these CaCl2 hexahydrate crystals? 

|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Wow, looks like solidifying raspberry syrup. Yummy!
Is cobalt formate hygroscopic, or could you grow crystals from it?
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
I just love these for their colour
Cobalt(II)orthomolybdate (left) and molybdatocobaltate(III) (right)
 
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Recrystallization is always mesmerizing to watch.
https://vimeo.com/470354647
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
My lab space represents!

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Red crystals of what is supposed to be ammonium pentachloroferrate forming as the solution cools. Plan is to let the beaker cool slowly on the cooling
hotplate overnight and to see whether I can recover dry crystals tomorrow. No idea if these are stable or hygroscopic. Will soon see!

|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Having uranium glass, fluorescein and a black light source I HAD to do it...
The decanter is full of fluorescein. So is the glass at the upper right corner. The one right below is empty.
The three other glasses were filled with drinks suited to the era this glass was made 
From left to right: Absynth, Absynth with water, Chartreuse.
(Yes, I used to smoke cloves and listen to The Cure)
The second picture is the decanter and the shot of fluorescein.
Closer to the light source they just saturate the sensor
 
[Edited on 24-10-2020 by Herr Haber]
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
I bought some calcium years back. But I never had occasion to open the tin. However, with the tin showing signs of age and one of my students asking
questions, I figured it was time for an unboxing. And to pop those babies under some oil.

|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Herr Haber, nice pictures! Maybe you would find it interesting to see if you can show fluorescerent quenching. When the concentration of a
fluorescerent molecule goes over a certain threshold, the fluorescence won't plateau, but actually go down because the dye will start to quench
itself.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Interesting idea.
I can see the difference between the glass and the dye but it's not as visible in the pictures. In the second one I diluted the original concentration
to about 1/4th. That's what you see in the second picture but they are also closer to the light source.
I was thinking I would try to dilute more. I dont know much about optics but found interesting that my 1W blue laser would totally scatter. Not even a
hint of blue on the other side of the decanter because of the dye.
Maybe I should try your suggestion in a beaker so the threshold is more visible when I make additions ?
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
You could make a dilution series indeed and check when the fluorescence goes down with rising concentration. It shouldn't take a lot.
|
|
|
MidLifeChemist
Hazard to Others
  
Posts: 192
Registered: 4-7-2019
Location: West Coast USA
Member Is Offline
Mood: precipitatory
|
|
Quote: Originally posted by j_sum1  | I bought some calcium years back. But I never had occasion to open the tin. However, with the tin showing signs of age and one of my students asking
questions, I figured it was time for an unboxing. And to pop those babies under some oil.
|
nice! how much calcium is that?
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by MidLifeChemist  | Quote: Originally posted by j_sum1  | I bought some calcium years back. But I never had occasion to open the tin. However, with the tin showing signs of age and one of my students asking
questions, I figured it was time for an unboxing. And to pop those babies under some oil.
|
nice! how much calcium is that? |
115 grams. It is pretty light stuff. And hard. Not sure what happens when I want a small piece.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
On a similar theme,
Lion850 arranged for me to score this little jar at a bargain price.
It is somewhat affected by air but a bit of hydrocarbon will prevent that from being an ongoing problem.
Standard size reference is given. There is also a lump of lead in there to keep it from floating.


|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
j_sum1 I like the "Standard size reference"; indeed universal hahaha.
I visited the supplier yesterday to get some thiourea; I meant to ask if he has more lithium but forgot.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
handsome lil borax crystal

al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
[rquote=647265&tid=26378
...I visited the supplier yesterday to get some thiourea... ...
[/rquote]
Out of curiosity, what would 10-50g of thiourea, shipped to the US, cost?
|
|
|
| Pages:
1
..
67
68
69
70
71
..
77 |