| Pages:
1
..
65
66
67
68
69
..
81 |
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
It looks correct to me. I am using CaCl as a descecator
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Is 46-0-0 fertilizer , pure Urea ? That's what the guy from fertilizer shop told me
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
I have neutralised HCLO4 to a ph of 7 using sodiumcarbonate (the hydrate), to regular household soda. Now I eveporated the solution and crystals
appear. Since i used a slight excess of carbonate, there is contamination of some sodiumcarbonate. I want to use this NaCLO4 to convert it to
Ammoniumperchlorate. I was thinking of using ammoniumchloride, since the sodiumchloride that will be formed is much more soluble then the
ammoniumperchlorate. I have also ammonium bicarbonate. Which route would be preferabme?
http://www.chlorates.exrockets.com/ammper1.html (found it)
[Edited on 23-1-2019 by snooby]
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Why you want to do it with the hard way ? Why you did not just neutralize your HCLO4 with ammonium bicarb to form directly ammoniumperchlorate ? You
can also bubble ammonia gas into Hclo4.
[Edited on 24-1-2019 by underground]
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Nitromethane Sensitizers
Anyone have any information for any easy to make/find NM sensitizer ?
|
|
|
MineMan
International Hazard
    
Posts: 1004
Registered: 29-3-2015
Member Is Offline
Mood: No Mood
|
|
Underground. I thought microballoons and al work fine... I take it you want a chemical sensitizer though??
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Not only a chemical sensitizer would be better but also i do not have access to microballons.
[Edited on 24-1-2019 by underground]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
https://www.amazon.com/Glass-Microspheres-1-Quart/dp/B00MNSE...
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
  
|
|
|
snooby
Hazard to Self
 
Posts: 88
Registered: 24-5-2013
Member Is Offline
Mood: No Mood
|
|
NaCLO4
Underground: yes a even better way to make AP is to neutralise HCLO4 with strong ammonia solution, this way you get pure AP.
HOWEVER: my plan was to make NaCLO4, not AP. At the last moment I received info that I could buy NaCLO4   . .
Back to the NaCLO4 synthesis, I still want to make a litle.bit of pure NaCLO4.
So now I have probably around 90% NaCLO4 with some NaCO3 (quite a lot:cool . How
can I purify this?? I was thinking to redisolve the NaCLO4/carbonate again, and then add diluted HCLO4 in it until no CO2 anymore is noticable. Then
again evaporate the very diluted HCLO4 / NaCLO4 solution is evaporated on hotplate. . How
can I purify this?? I was thinking to redisolve the NaCLO4/carbonate again, and then add diluted HCLO4 in it until no CO2 anymore is noticable. Then
again evaporate the very diluted HCLO4 / NaCLO4 solution is evaporated on hotplate.
However: is this safe to do?? Can I just evaporate this without problems. I gues its just the azeotrope of HCLO4 will evaporate with the water, but I
just want to be SURE. Furthermore, which temp does this be done..? I cant find evaporation point of diluted perch acid (only 70% is around 203C)...
So wich temp should i use to eveporate remaining acid (10percent hclo4), or just not do it?
Edit edit.. Should have figure this out earlier:
Solubility of Na2CO3 compared to NaCLO4
At 1 degrees C: 7 grams of Na2CO3 in 100 ML H2O
167 grams of NaCLO4 in 100 ML H2O
Done..
Sorry for lots of messages but I just like to figure this kind of stuff out myself, I have had only veryyy basic science on high-school
[Edited on 25-1-2019 by snooby]
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
An acid, a strong base. Urbanski has all the ideas.
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Nitromethane gel on cap No.8 sensitive:
Nitromethane.....................................80%
commerce nitrocellulose (12,4N).......10%...(best is shotgun NC propellant)
Aluminium bright color type................5%....(or Mg, Magnalium, any Al)
Glass microballoons...........................2%....(best is 125g/liter type)
Variable contents for NC....................3%+............ ..................LL ..................LL
[Edited on 25-1-2019 by Laboratory of Liptakov]
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
NaClO4
@snooby : You have HClO4?....Thus you can prepare anything. KClO4, NaClO4, NH4ClO4, LiClO4 and lot nexts. You leave the think on boiling dillute
HClO4. All around 10 meters will incredible oxidized from steam. And if you will have NaClO4 is it also almost all, what you need.....
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Will this ball mill do the job for Black Powder and aluminium ?
Im not sure cause of that shape of the drum
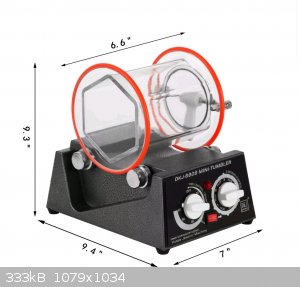
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by Laboratory of Liptakov  | Nitromethane.....................................80%
commerce nitrocellulose (12,4N).......10%...(best is shotgun NC propellant)
Aluminium bright color type................5%....(or Mg, Magnalium, any Al)
Glass microballoons...........................2%....(best is 125g/liter type)
Variable contents for NC....................3%+............ ..................LL ..................LL
[Edited on 25-1-2019 by Laboratory of Liptakov] |
Laboratory of Liptakov is it storage stable that mixture or you made it before use ?
It should work
Note that
Critical speed = 265.45 / sqrt (jar ID - media diameter) (diameters in inches)
Optimal speed is 65% of the critical speed.
[Edited on 25-1-2019 by underground]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Planning to write sort of an more in depth essay about the copper/ascorbic reduction with some additional information about the literature concerning
the selective mono reduction of some polynitroarenes. Does anyone know if mono reduction of 2,4,6-trinitroanisole has been attempted and the resulting
isomer distrubution for the amine?
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
@underground ....Nitromethane gel is stable. Is nothing what should by reacting together there. Storage in close jar, of course. Against evaporation
of NM.........
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by FeedMe94  | Will this ball mill do the job for Black Powder and aluminium ?
Im not sure cause of that shape of the drum[/rquote]
It should work
Note that
Critical speed = 265.45 / sqrt (jar ID - media diameter) (diameters in inches)
Optimal speed is 65% of the critical speed.
[Edited on 25-1-2019 by underground] |
Any idea what media to use? Im trying to find Lead Antimony spheres but the only thing i can find is small metal balls used as ammunition. Also found
Alumina ceramic balls. The company says no sparks can be produced
Edit*
My jar is 3 litres. I need 1.5 litres of milling media ?
[Edited on 26-1-2019 by FeedMe94]
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Quote: Originally posted by FeedMe94  |
Any idea what media to use? Im trying to find Lead Antimony spheres but the only thing i can find is small metal balls used as ammunition. Also found
Alumina ceramic balls. The company says no sparks can be produced
Edit*
My jar is 3 litres. I need 1.5 litres of milling media ?
|
Yea you are going to need 1,5 litres of media.
By the way, why don't you try to make one on your own, it shouldn't be hard to make it and it would be much more better and cheaper. Thick pvc pipe
will do the job as a jar.
Thanks
P.S. Laboratory of Liptakov i have seen you have made some plastics with scapa P.I.B and 5/40 motor oil with very good results. Have you ever try it
with methyl ricinoleate instead of 5/40 motor oil ?
[Edited on 26-1-2019 by underground]
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Can organic perchlorates like HDP or Perchloric acid be made fromm POTASSIUM Perchlorate without distilation ?
|
|
|
C6(NO2)5CH2CH(CH3)N(NO2)2
Harmless

Posts: 43
Registered: 4-4-2018
Member Is Offline
Mood: No Mood
|
|
loading and pressing powder in an irregular hole
suppose you had an irregularly shaped cavity ~1cm wide by 10cm long that you wanted to put an explosive powder in, but the only hole to access it was
5-8mm wide. What would be the best way to pack the powder inside? I can't pour a powder in and fill the voids with a liquid-phase explosive, because
of chemical compatibility issues with the walls of the container. So, what's the best way to press a powder to a high density when you only have a
small hole to introduce it through?
Put that in your pipe and smoke it!
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Melted ETN, then allow it to cool
[Edited on 6-2-2019 by underground]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
(edit)
sorry I was looking for the general-purpose short questions thread
[Edited on 8-3-2019 by mayko]
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
Is there any soluble hexamine salt ? (In order to use for metathesis reaction for HDN /HDP production )
I can not find any info from net
|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
Anyone done the cyanamide-hydrazine aminoguanidine synthesis?
Just tried it but got no/ negligible yield.
I know my Calcium Cyanamide was decent quality, and my Hydrazine Sulfate was as well at some point (bottle is two years old, however the sample has
become quite acidic).
Problems emerged when the pH would not increase substantially during the reaction, as it should with formation of free base Aminoguanidine.
I also accidentally let the thermometer come out of the solution, so the temperature increased to boiling for several minutes.
The Bicarbonate was added as a mostly-dissolved slurry. It all dissolved in the filtered reaction mixture and effervescence persisted for several
minutes.
No precipitate formed.
I'm stuck. Any ideas where this could have gone wrong?
|
|
|
| Pages:
1
..
65
66
67
68
69
..
81 |