| Pages:
1
..
65
66
67
68
69
..
77 |
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Some lovely cultures of marine fungi
(source: https://www.cell.com/current-biology/fulltext/S0960-9822(19)31100-5 )

al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Some crystals of hexamine that formed in a flask I neglected to clean for a couple weeks

|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Ammonium chloride that crystallized out beautifully overnight after postponing the wash up of this 2L seperatory funnel.

[Edited on 28-5-2020 by Sigmatropic]
|
|
|
Morgan
International Hazard
    
Posts: 1694
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Those NH4Cl crystals almost look like or are reminiscent of the gradation markings on a flask. Nice straight lines and effect, something like that
might be a good contender in a photo contest.
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
F---- Yeah baby... Sorry I'm excited.



Now all the fun of coming up with the funding for measurement devices, till then, these gonna hide on a shelf.. I loved the service, wonderful and
easy
USA shipping only ... http://www.minresco.com/index.html
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
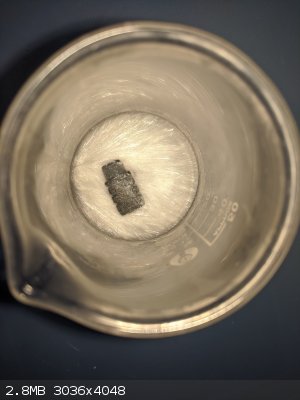
Some lead acetate crystals that I recently produced with some scrap lead.
|
|
|
Boffis
International Hazard
    
Posts: 1867
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@violet sin; is that tyuyamunite I see at the bottom?
Now these are tyuyamunite:

Tyuyamunite encrusted blocks underground in the Shekafter East Mine, Kirgyzstan

Close up of a 3" specimen
 I worked on uranium exploration for quite a few years. I even worked right next
to the old Tyuya Muyun Mine near Yaravan in southern Kirgyzstan and managed to collect tyuyamunite, turanite etc from this classic site. I worked on uranium exploration for quite a few years. I even worked right next
to the old Tyuya Muyun Mine near Yaravan in southern Kirgyzstan and managed to collect tyuyamunite, turanite etc from this classic site.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
A freshly cleaned fume hood, ready to be messed up again!

|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Boffis... PRETTY, WOW!!! Yes my sample was one of the low cost Tyuyamunit samples from the link. How fantastic! You actually worked there, bet
you've seen some breathtaking mineralizations.
The detection equipment is beyond me at the moment. I'm trying to figure out the best approach on a budget, to have the most situational
awareness. There are some places I've found deer hunting with sulfides in quartz veins, deep black/brown colored rusty crud, and generally
interesting appearance. I'd love to check them out.
California has some radioactive minerals around. Could be rewarding to check creeks and rivers near granite, for placer thorianite. Till then
I'm blind to my surrounding in that regard.
I fancy the idea of making a cloud chamber, and have been reading up in the radioactive section here. Any tips or suggestions on equipment are
welcome.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Chlorophyll in acetone (visible, visible+NIR, NIR, NIR)
(I fucked up the focus while hacking the camera sensor of this phone)
   
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Good old Ark-In-Saw. This cloud teased me, but never actually dropped a funnel.

"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
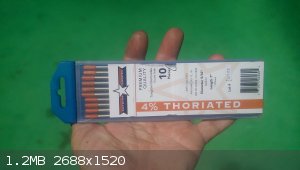
I got a couple element samples, one kinda.
The TIG electrodes are 4% ThO2 instead of the normal RED tip that have 2%. Amazon purchase at a little over 50$ but ~41.25g each x 10 isn't a bad
amount of sample for the price.
The 100g Gadolinium was an aliexpress purchase ~35$ after shipping/tax. There is a seller of ThO2 white as the driven snow on aliexpress... But
between the mineral sample I have, couple small harbor freight TIG electrodes and the fine , shiny and richer samples ... No need to go there.
on its way still is a wish purchase of 100g Gallium for ~36$ from China. Probably take a while to get here. 1g of germanium that was only $5 but
shipping brought it up to $8 and change, was not paying attention.
Also I finally got around to epoxy coating my asbestos samples. White asbestos in serpentine, so I'm pretty sure it's crysoltile. Fills all the
cracks in serpentine, comes in tan/honey/light blue/white colors. Good stuff to get locked down, don't care if it downgraded the specimens. The best
bleached rock, got the next batch of epoxy, which was far more yellow, and almost looks worse than the poorly cleaned rocks and clearer epoxy... A lye
/ peroxide mix was used. The bucket they were in gave way to sunlight and leaves fell in to get a nice adherent organic film. No acid was used.
 
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
how are you going to separate the ThO2 from the tungsten? hydrogen peroxide digestion or another way?
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
violet sin
International Hazard
    
Posts: 1480
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
Ahhh, my lab has been packed up for a couple years. Once upon a time pre-children, that was my goal, I wanted the thorium dioxide out. My sample
then was small and expensive. IIRC it was ~$15 for a 3 pack of thorianated tungsten TIG electrodes at harbor freight. They look like crappy little
black needles and there was little to them... Returns would have been small. If I ever did dissolve one it was 40% of one rod, but I'm pretty sure
it went to first attempts at NO2 via high temp arc.
Peroxide works just fine for all the incandescent filaments previously solved. I'm sure plenty of members have dissolved the TIG rods and can give
you pointers.
Right now I'm far more of a collector/builder that wet chem in any sense. Expanding things for when I've more time, less worry.
1) Nope, have all the old tug rod, none was digested. Guess it was a 5 pack.
2) Potassium carbonate from years back, done here on SM from banana peels...
3) Copper sulfamate possibly Zinc contamination, but was third re-xtal after picking blue away from clear crystals.
4) potassium iron oxalate crystals from when I prepared them here years ago... Aged well with no top eh?
   
|
|
|
OldNubbins
Hazard to Others
  
Posts: 136
Registered: 2-2-2017
Location: CA
Member Is Offline
Mood: Comfortably Numb
|
|
Good friend gave me this jar about 6 months before he passed. Told me, "Here, can you do anything with this?". I thought he was looking for a laugh as
he watched me go giddy over an empty jar. Nope, wasn't empty. 
[Edited on 6-7-2020 by OldNubbins]

[Edited on 6-7-2020 by OldNubbins]

|
|
|
sciece nerd
Harmless

Posts: 25
Registered: 27-8-2019
Member Is Offline
|
|
What's that?
(The label looks like "astanum", which is apparently not a thing)
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I think you will find it says, "Platinum".
Yum!
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
To me it says clearly platinum too.
Reminds me of the "710 cap" joke 
|
|
|
sciece nerd
Harmless

Posts: 25
Registered: 27-8-2019
Member Is Offline
|
|
I guess I have to look more closely.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I got glasses a few years ago, and wow!
Didn't remembered the world to be that detailed anymore 
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
So I made some potassium bis(oxalato)cuprate(II), and got some gorgeous blue needles. I also got a bit of precipitate that looked like copper(II)
hydroxide, so I added excess potassium oxalate and recrystallized that. That gave me some lighter blue, differently shaped crystals. Not
sure what they are- possibly an oxalato/hydroxy complex?
Aha! According to https://www.coursehero.com/file/26392719/lab-03pdf/ , the needles are the tetrahydrate, and the blocks are the dihydrate.
[Edited on 16-6-2020 by DraconicAcid]
  
[Edited on 16-6-2020 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Bedlasky
International Hazard
    
Posts: 1239
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
These blue needles looks awesome!
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Interesting color I thought for a copper salt (that does not contain another metal, only copper). A dark purple copper cyanurate. Procedure was
described in more detail here: http://www.sciencemadness.org/talk/viewthread.php?tid=155624

|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
KCl fractional crystallization from low sodium salt. I dissolved the salt in boiling water until it started to crystallize out added a little to
dissolve it again, then I filtered the silicon dioxide additive and left it on the table to cool, and later put it in the freezer.
Gonna collect crystals, concentrate it to saturation and repeat. I suppose NaCl (30%) will also start to crystallize eventually so likely the first
batch could be the only pure KCl.

|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
I made some propyl po-toluate, and it seems to be crystallizing nicely (either that or, there's a lot of unreacted acid that's decided to
crystallize).

Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
| Pages:
1
..
65
66
67
68
69
..
77 |