| Pages:
1
..
61
62
63
64
65
..
81 |
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Not too hard. The set up looks like the one in the picture. Collect the fraction from about 118 degrees C to 124 degrees C. This is your azeotropic
nitric acid. I think you could also do a simple distillation here if you go slowly.
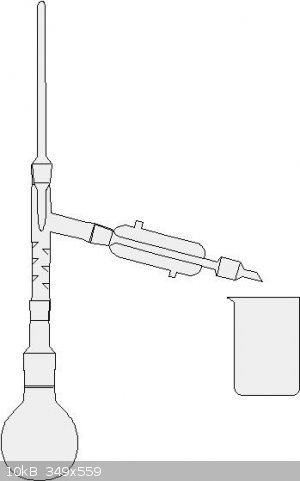
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by CobaltChloride  | | Not too hard. The set up looks like the one in the picture. Collect the fraction from about 118 degrees C to 124 degrees C. This is your azeotropic
nitric acid. I think you could also do a simple distillation here if you go slowly. |
Thank you for responding. 
Have you done this before?
Sorry to abuse your good will, but what are the approximate yield?
I'm asking because azeotropic nitric acid got very hard to get here where I live, but for some reason the 53 % concentration is very, very cheap and
sold OTC with no limit for how much you can buy.
I'm not going to buy gallons of it, but instead I want to make it more economically viable to experiment since I work at a shitty job and most of my
money goes to pay my college bills.
Realistically, much much 68% nitric acid would be obtained from a 5 liters bottle of the 53 % one? I'm not talking about theoretical yields though.
Oh, hello!  |
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
Do you have access to 98% Sulfuric acid?
If so you could use the same distillation setup with a water-cooled liebig condenser and a 2:1 ratio of H2SO4/HNO3 in the distilling flask.
The sulphuric acid will hold the water and around 90% red fuming nitric acid will distill over.
You can then dilute this to 70% or whatever concentration you want by addition of water.
Be good, otherwise be good at it 
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Quote: Originally posted by joseph6355  | Quote: Originally posted by CobaltChloride  | | Not too hard. The set up looks like the one in the picture. Collect the fraction from about 118 degrees C to 124 degrees C. This is your azeotropic
nitric acid. I think you could also do a simple distillation here if you go slowly. |
Thank you for responding. 
Have you done this before?
Sorry to abuse your good will, but what are the approximate yield?
I'm asking because azeotropic nitric acid got very hard to get here where I live, but for some reason the 53 % concentration is very, very cheap and
sold OTC with no limit for how much you can buy.
I'm not going to buy gallons of it, but instead I want to make it more economically viable to experiment since I work at a shitty job and most of my
money goes to pay my college bills.
Realistically, much much 68% nitric acid would be obtained from a 5 liters bottle of the 53 % one? I'm not talking about theoretical yields though.
|
I haven't done it yet as I don't need to in order to get nitric acid, but the procedure was taken from NileRed's video on making azeotropic nitric
acid (https://www.youtube.com/watch?v=KBeo8nww21g&t=312s). More specifically, from the purification step at 5:25. You'd theoretically get about 3.68
liters of acid after distillation.
[Edited on 1-4-2018 by CobaltChloride]
|
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
Quote: Originally posted by greenlight  | Do you have access to 98% Sulfuric acid?
If so you could use the same distillation setup with a water-cooled liebig condenser and a 2:1 ratio of H2SO4/HNO3 in the distilling flask.
The sulphuric acid will hold the water and around 90% red fuming nitric acid will distill over.
You can then dilute this to 70% or whatever concentration you want by addition of water.
|
This method has the disadvantage of producing nitric acid of less accurate concentration though.
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by greenlight  | Do you have access to 98% Sulfuric acid?
If so you could use the same distillation setup with a water-cooled liebig condenser and a 2:1 ratio of H2SO4/HNO3 in the distilling flask.
The sulphuric acid will hold the water and around 90% red fuming nitric acid will distill over.
You can then dilute this to 70% or whatever concentration you want by addition of water.
|
I thought about that. Honestly it isn't very economically efficient since 98% h2so4 isn't cheap.
I'm also kind of scare of the fuming nitric acid. Its very corrosive and almost everything it touches catches on fire instantly, including my cheap
gloves that are rated for common acids.
Quote: Originally posted by CobaltChloride  | Quote: Originally posted by joseph6355  | Quote: Originally posted by CobaltChloride  | | Not too hard. The set up looks like the one in the picture. Collect the fraction from about 118 degrees C to 124 degrees C. This is your azeotropic
nitric acid. I think you could also do a simple distillation here if you go slowly. |
Thank you for responding. 
Have you done this before?
Sorry to abuse your good will, but what are the approximate yield?
I'm asking because azeotropic nitric acid got very hard to get here where I live, but for some reason the 53 % concentration is very, very cheap and
sold OTC with no limit for how much you can buy.
I'm not going to buy gallons of it, but instead I want to make it more economically viable to experiment since I work at a shitty job and most of my
money goes to pay my college bills.
Realistically, much much 68% nitric acid would be obtained from a 5 liters bottle of the 53 % one? I'm not talking about theoretical yields though.
|
I haven't done it yet as I don't need to in order to get nitric acid, but the procedure was taken from NileRed's video on making azeotropic nitric
acid (https://www.youtube.com/watch?v=KBeo8nww21g&t=312s). More specifically, from the purification step at 5:25. You'd theoretically get about 3.68
liters of acid after distillation.
[Edited on 1-4-2018 by CobaltChloride] |
I watched the video before posting the question.
Are you certain that I'll get 68% nitric at the end flask?
Thinking long term, its better for me to buy good quality glassware that will allow me to concentrate the acid instead of buying directly.
As I said, 30 USD for 5 liters of 53% that will yield somewhat on the 3 liter range would be very good since 1 L 68% NA costs about 40 USD alone here,
and you also go to a watchlist. I don't like watchlists.
[Edited on 1/4/18 by joseph6355]
Oh, hello!  |
|
|
CobaltChloride
Hazard to Others
  
Posts: 239
Registered: 3-3-2018
Location: Romania
Member Is Offline
|
|
I agree with the fact that it's better to buy good quality glassware rather than buying the nitric acid directly. I suggest looking at the seller
deschem on eBay if you're interested in buying glassware. Since NileRed's acid was even more dilute than your acid and he did get 68% nitric acid in
the receiving flask, you should also get 68%. If you want some proof it is at that concentration, do as NileRed did and measure its density.
|
|
|
greenlight
National Hazard
   
Posts: 737
Registered: 3-11-2014
Member Is Offline
Mood: Energetic
|
|
I was just about to write that you can get a fairly accurate idea of the concentration using the density. 
Heres a calculator which takes into account ambient temperature as well:
http://www.handymath.com/cgi-bin/nitrictble2.cgi?submit=Entr...
Be good, otherwise be good at it 
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Thank you! 
Oh, hello!  |
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Is recrystallizing trinitrophenol from non-distilled water something stupid? Will it form its metal picrates from the ions in the water? Is the amount
formed dangerous?
Oh, hello!  |
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Hello , im looking for some alternative fuels for Ammonium nitrate. So far i tried petrol , hexamine and wax. Any other fuel easy obtainable ?
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Quote: Originally posted by feedme94  | | Hello , im looking for some alternative fuels for Ammonium nitrate. So far i tried petrol , hexamine and wax. Any other fuel easy obtainable ?
|
Isnt AN the oxidizer and petrol the fuel?
There are other nitrate salts that are easily available.
Regarding fuel alternatives, you could try methane, oil (vegetable, mineral oil and fuel oil), kerosene... Some of them aren't ideal but they could be
used.
If you are aiming for balanced OB, you would need to change the proportions for each different fuel and mix the stoichiometric amount.
What is the final product that you are trying to obtain?
[Edited on 10/4/18 by joseph6355]
Oh, hello!  |
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by joseph6355  | Quote: Originally posted by feedme94  | | Hello , im looking for some alternative fuels for Ammonium nitrate. So far i tried petrol , hexamine and wax. Any other fuel easy obtainable ?
|
Isnt AN the oxidizer and petrol the fuel?
There are other nitrate salts that are easily available.
Regarding fuel alternatives, you could try methane, oil (vegetable, mineral oil and fuel oil), kerosene... Some of them aren't ideal but they could be
used.
If you are aiming for balanced OB, you would need to change the proportions for each different fuel and mix the stoichiometric amount.
What is the final product that you are trying to obtain?
[Edited on 10/4/18 by joseph6355] |
Im testing some ANFO charges. The best so far is AN with Hexamine but im using it as booster for AN with Wax. Im looking for something to replace the
wax. Kerosene or fuel oil doesnt seem good cause of their cooling effect while evaporate
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
The 3-nitrogroup of 2,3,4,6-tetranitroaniline is easily displaced by nucleophiles:
1. Reaction with water results in 3-hydroxy 2,4,6 trinitroaniline (Chemistry of powder and explosives, T.L Davis, page 173)
2. Reaction with alcoholic ammonia results in 3-amino 2,4,6 trinitroaniline (Chemistry of powder and explosives, T.L Davis, page 173)
2. Reaction with azide results in 3-azido 2,4,6 trinitroaniline (which can rearrange to a furoxan)
3. Reaction with methanolic hydroxylamine results in 5-amino-4,6-dinitrobenzofuroxan (https://doi.org/10.1023/A:1023049531550)
How would 2,3,4,6-tetranitroaniline react with alcoholic hydrazine? Any ideas? Presumably, 3-hydrazino 2,4,6 trinitroaniline would form first, would
it rearrange internally or would it react preferably with the 3-nitrogroup of another 2,3,4,6-tetranitroaniline molecule?
|
|
|
underground
National Hazard
   
Posts: 702
Registered: 10-10-2013
Location: Europe
Member Is Offline
|
|
EM Substitute for experimenting with plasticizers
What easily available substitute can i use instead ETN/PETN for doing some tests with various plasticizers ? (plasticizers from gum/rat glue)
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Today i tested 4 different types of flash powder.
Kclo4 / Al 7 / 3
Kclo4 / Al /S 7 / 2 / 1
Kclo3 / Al 7 / 3
Kclo3 / Al /S 7 / 2 / 1
kclo4 is way more stable. Non of them, even with S wont go bang with impact on metal surface.
From the other side both kclo3 will go bang on impact, just the one with S a bit easier. All of them i think sounds the same. Maybe the kclo3 / al /
s sounded more like a thunder. I think it was faster
Any other ratios that might work better ?
|
|
|
Dornier 335A
Hazard to Others
  
Posts: 231
Registered: 10-5-2013
Location: Northern Europe
Member Is Offline
Mood: No Mood
|
|
This might interest you:

It's from Takeo Shimizu's book "Fireworks, the Art Science and Technique".
He claims the loudest combination of the three components is 64% KClO4, 23% dark Al and 13% S.
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Thank you so much Dornier !!! Not only helped me to achieve what im looking for (noise) but also you helped me save a lot of KCLO4 and Dark Al which
are expensive unlike Sulfur. Gonna try it soon
|
|
|
Rocinante
Hazard to Others
  
Posts: 121
Registered: 13-11-2017
Member Is Offline
Mood: No Mood
|
|
...falsified by direct pressure measurements (thin paper tubes), unfortunately, KClO4/Al is better than any other non-exotic mix - and much safer
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
Another question. Can i store 65% HNO3 , H2SO4 and 31-33% HCl in PET Plastic bottles ?They are brown but you can see through them
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Quote: Originally posted by feedme94  | | Another question. Can i store 65% HNO3 , H2SO4 and 31-33% HCl in PET Plastic bottles ?They are brown but you can see through them
|
No. PET is a polyester plastic, and strong mineral acids will cause it to break down.
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
What is the best plastic to store those acids , i dont really want to use glass as it breaks easily
[Edited on 28-4-2018 by feedme94]
|
|
|
Laboratory of Liptakov
International Hazard
    
Posts: 1387
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
Plast from bleach from store container is pretty resistant. Even against chlorine and chlorine oxides. Tested in chlorate cell device. Else plast were
dissolved. For example for electric uses.
Development of primarily - secondary substances CHP (2015) Lithex (2022) Brightelite (2023) Nitrocelite and KC primer (2024)
|
|
|
FeedMe94
Hazard to Self
 
Posts: 87
Registered: 1-4-2017
Member Is Offline
Mood: No Mood
|
|
We know what type it is ? Because i want to buy little 200ml bottles so i can handle them. The one that i have them now are 5L bottles
|
|
|
c1der
Harmless

Posts: 2
Registered: 29-4-2018
Member Is Offline
Mood: No Mood
|
|
Determination of 2,4,6-trinitrophenol or 2,4-dinitrophenol
Is there a "home chemistry" way to determine if my final product from ASA sulfonation and nitration is 2,4,6-trinitrophenol and not 2,4-dinitrophenol?
|
|
|
| Pages:
1
..
61
62
63
64
65
..
81 |