| Pages:
1
..
4
5
6
7 |
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
I kind of garbled words there, sorry. I did pretty much mean regular short-path vacuum distillation.
A rotovap could be used to remove solvent. It would probably be a bit of a push to try to use it to distill something like tryptamine that is solid at
ambient temperature, though.
|
|
|
Quatro
Harmless

Posts: 22
Registered: 31-10-2015
Location: Arizona
Member Is Offline
Mood: Nucleophillic
|
|
Well yes, the Rotovap would be used to remove the acetophenone from the tryptamine/acetophenone mixture. Why would you want to distil pure Tryptamine?
Or do you mean, if you hypothetically needed to remove it from something with an even higher boiling point?
I'll probably just try regular vacuum distillation, I don't have a Rotovap but that would be a neat piece of equipment to own!
|
|
|
hive3
Harmless

Posts: 27
Registered: 3-7-2013
Member Is Offline
Mood: No Mood
|
|
Acetophenome Decarb Procedure
These are my notes from an Acetophenome decarb. I am planning on dissolving the product in dry acetone to and gassing with co2 to complete the
purification.
I am posting it now because it provides some vacuum and temperature reading that have not appeared before.
1cm stir bar, 9.5g Tryptamine and 35ml of acetophenome is placed in a single neck 100ml flask with 14/20 Glassware for distillation with leibig,
vacuum trap, bubbler, 50 ml receiver flask , and hot oil bath.
3:15pm Oil Bath set to 133C solution hits 121c and bubbling commences at 1 bubble per second.
3:35pm increase oil bath to 137c because bubbling is dropping to 1.5 bubbles per second. Solution at 121C.
4:08 bubbling stops and solution becomes a clear amber oil.
Remove bubbler and connect to aspirator at 44mBar. Oil bath set to 128C.
4:40 distillation head temp reads 95C and Acetophenome and a nonmisicable fluid come over.
4:48 oil bath increased to 135C and distillation slows and stops. About 25ml of acetophenome are recovered.
Leibig and receiver flask are removed and replaced with bump trap connected to a M-M elbow connected to a 2 neck 100Ml flask with the second neck
connected to an A/C vacuum pump. The oil bath is removed, and the boiling flask place directly on a hot plate. Vacuum at 2mbar is applied and heating
is commenced.
The remaining solution begins to boil and the fraction from 145C to approximately 157C is discarded. It is (a combination of Acetophenome and
Tryptamine?) approximately 10ml. At the upper temperature a thick amber oil starts to condense on the sides of the bump trap bulb rather than dripping
down to the bottom of the bulb. A new flask is attached and distillation is continued. The remaining boiling oil bumps considerably even with stirring
requiring the bump trap. The bump trap has to be tented and heated with a heat gun to keep the condensing oil from plugging everything up. It pretty
much solidifies on condensation.
The boiling tryptamine oil gets darker as distillation continues, and distillation is stopped when the distillate starts getting darker too.
To be continued.
|
|
|
FormicAcidAnimal
Harmless

Posts: 9
Registered: 29-10-2015
Member Is Offline
Mood: No Mood
|
|
I've done this several times getting 65-80% yield in 50-100g scale with 100% OTC chemicals
A few hundret mL turpentine (the good old classic genuine stuff, not a substitute), a few mL of essential oils of Mentha crispa ("Krauseminzöl", very
cheap from Ebay.de, contains mostly carvone) and 100g L-tryptophane food greade where refluxed in a 2l RBF until CO2 evolution isn't visible anymore
(2-3h, depending on the amount of catalyst used). The turpentine was extractet three times with ~5% AcOH while still hot and aqueous solution was
extracted a few times with DCM or EtOAc to remove the essential oil.
Large excess of 50% NaOH solution was added to the aqueous phase until an oil begins to seperate. Afte some more stirring an scratching the oil begins
to cristallise to sticky dirty-amber-colored chunks.
The chunks where filtered, washed with thinned aq. ammonia and dest. water and dried, giving a ~90+% yield of crude tryptamine base.
The crude trypamine was short path destilled using good water-jet vacuum or better vacuum source, leaving a lot of dark-brown sticky dirt/tar, giving
nice slightly amber colored tryptamine base, which cristallises spontaneously after a few minutes to give 65-80% overall yield. No
byproducts/completly pure according to TLC
Usual A/B cleanup for amines doesnt well work at all for tryptamine in my experience, as the base is only barely soluble in most usual organic
solvents, while the salts are also partially bad solouble in water (but slightly soluble in organic solvent) and a lot of the dirt seems to also have
basic groups - at least I haven't managed to get are even baraley pure-enough product using method. .
An alternative might to dissolve the crude trypamine in dry EtOH, filter it with decolorizing carbon and gas it with dry CO2 using a "Sodastream
Crystal" etc. to form the insoluble carbamate (US2943093), filter the carbamat and add water or NaOH to liberate the freebase again, let the freebase
cristallize again, filter it and wash it with dest. water.
I haven't tried this route though and it don't know if the purity optained is as good as when using short path destillation, but if you wan't to avoid
vacuum destillation that would be the way I would try.
[Edited on 24-11-2015 by FormicAcidAnimal]
|
|
|
DrMethyl
Harmless

Posts: 34
Registered: 23-11-2015
Member Is Offline
Mood: No Mood
|
|
The non-miscible liquid you noticed is probably water isssued from the condensation of tryptamine with acetophenone to give the imine.
Imine formation is a big problem to remove the acetophenone by distillation. Did you check the mp of your final product because I think it might be
seriously contaminated with the condensation product.
It happened to me and I had to hydrolises it and exctrat the tryptamine via acid/base.
|
|
|
hive3
Harmless

Posts: 27
Registered: 3-7-2013
Member Is Offline
Mood: No Mood
|
|
Acetophenome Workup
What acid and base did you use in your workup? I did try a separation directly from Acetophenome using dilute HCL but ended with a black sludgy mess.
The distillation gives the same thick amber oil that I have gotten from the Turpentine and Mineral oil processes but does not crystalize as stated in
some of the earlier posts. I still have the result under nitrogen awaiting final workup.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
I can sell you a nice one. If you're interested send me a u2u.
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
yanzi
Harmless

Posts: 16
Registered: 16-1-2016
Member Is Offline
Mood: No Mood
|
|
I ordered 100 mL of 2-cyclohexenone from MP (pm me for full name -- it's a perfectly fine lab chemical supply site, kind of like an alibaba for
chemical suppliers, I just don't want this route shut down). I would be willing to sell some of the catalyst in smaller batches after it arrives later
today 
My main issue with decarboxylation is solubility. For example, I have been able to source some carbidopa from an Indian pharmacy. As you can see, if
you methylenate it (per Rhodium) with dichloromethane, tetrabutylammonium bromide PTC catalyst (I should have bought the iodide, but w/e) and sodium
iodide catalyst (http://onlinelibrary.wiley.com/doi/10.1002/jccs.199100038/ab... -- I have the full text on how this enhances the reactivity of alkyl halides in
PTCs), then decarboxylate it in cyclohexanol I should be able to get a certain entactogenic product. 
My problem is that carbidopa, as well as its derivatives that are also amino acids, is weakly soluble in water and even less soluble in DCM. So it's
hard to do a typical acid-base extraction. If I run a PTC as per Rhodium's article "the methylenation of catechol" and end up with methylenated
carbidopa, I still have to worry about the zwitterionic nature of the intermediate. One patent suggests I could use extract amino acids with a ~1M PTC
catalyst (like Aliquat 336, but I have TBAB) in an organic layer to exchange with a monoanionic form of the amino acid. NaBr / NaI would get left
behind in the aqueous phase and/or precipitate in the organic phase, while a lipophilic dimer of tetrabutylammonium [amino acid anion] would get
extracted into an organic layer. I could then back-extract into acidic water?
[Edited on 16-1-2016 by yanzi]
|
|
|
FormicAcidAnimal
Harmless

Posts: 9
Registered: 29-10-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by yanzi  | I ordered 100 mL of 2-cyclohexenone from MP (pm me for full name -- it's a perfectly fine lab chemical supply site, kind of like an alibaba for
chemical suppliers, I just don't want this route shut down). I would be willing to sell some of the catalyst in smaller batches after it arrives later
today 
My main issue with decarboxylation is solubility. For example, I have been able to source some carbidopa from an Indian pharmacy. As you can see, if
you methylenate it (per Rhodium) with dichloromethane, tetrabutylammonium bromide PTC catalyst (I should have bought the iodide, but w/e) and sodium
iodide catalyst (http://onlinelibrary.wiley.com/doi/10.1002/jccs.199100038/ab... -- I have the full text on how this enhances the reactivity of alkyl halides in
PTCs), then decarboxylate it in cyclohexanol I should be able to get a certain entactogenic product. 
My problem is that carbidopa, as well as its derivatives that are also amino acids, is weakly soluble in water and even less soluble in DCM. So it's
hard to do a typical acid-base extraction. If I run a PTC as per Rhodium's article "the methylenation of catechol" and end up with methylenated
carbidopa, I still have to worry about the zwitterionic nature of the intermediate. One patent suggests I could use extract amino acids with a ~1M PTC
catalyst (like Aliquat 336, but I have TBAB) in an organic layer to exchange with a monoanionic form of the amino acid. NaBr / NaI would get left
behind in the aqueous phase and/or precipitate in the organic phase, while a lipophilic dimer of tetrabutylammonium [amino acid anion] would get
extracted into an organic layer. I could then back-extract into acidic water?
[Edited on 16-1-2016 by yanzi] |
I would try to make diiodomethane first from DCM and use it as solvent (bp 181°C - should dissolve it much better and also highly increase reaction
rates) or making water solouble phosphate salt from it or simply use DMF as solvent . - btw. how you do you plan to get rid of the -NH2-Group of
Carbidopa?
|
|
|
Scr0t
Hazard to Others
  
Posts: 118
Registered: 14-1-2012
Location: Europe
Member Is Offline
Mood: Desiccated
|
|
| Quote: |
I have been able to source some carbidopa from an Indian pharmacy. As you can see, if you methylenate it (per Rhodium) with dichloromethane,
tetrabutylammonium bromide PTC catalyst (I should have bought the iodide, but w/e) and sodium iodide catalyst (http://onlinelibrary.wiley.com/doi/10.1002/jccs.199100038/ab... -- I have the full text on how this enhances the reactivity of alkyl halides in
PTCs), then decarboxylate it |
You'll probably get alkylation on the nitrogen atoms too. You'll only get an unworkable mess by doing this.
[Edited on 23-1-2016 by Scr0t]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
CH2I2 only reacts with the strongest nucleophiles; if you read Bonthrone and Cornforth's seminal 1964 article on methylenation it explains that the
methylene halides will only react readily with the catecholate dianion (not the monoanion). Amine alkylation won' t be an issue but you may have some
difficulty stabilizing the trianion (since COOH is also deprotonated) in aqueous media. You could (I think?) use NaOEt/EtOH and you won't need any
PTC, but there is a side reaction creating diethoxymethane (an interesting compound, but not the goal).
[Edited on 24-1-2016 by clearly_not_atara]
|
|
|
yanzi
Harmless

Posts: 16
Registered: 16-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by FormicAcidAnimal  | Quote: Originally posted by yanzi  | I ordered 100 mL of 2-cyclohexenone from MP (pm me for full name -- it's a perfectly fine lab chemical supply site, kind of like an alibaba for
chemical suppliers, I just don't want this route shut down). I would be willing to sell some of the catalyst in smaller batches after it arrives later
today 
My main issue with decarboxylation is solubility. For example, I have been able to source some carbidopa from an Indian pharmacy. As you can see, if
you methylenate it (per Rhodium) with dichloromethane, tetrabutylammonium bromide PTC catalyst (I should have bought the iodide, but w/e) and sodium
iodide catalyst (http://onlinelibrary.wiley.com/doi/10.1002/jccs.199100038/ab... -- I have the full text on how this enhances the reactivity of alkyl halides in
PTCs), then decarboxylate it in cyclohexanol I should be able to get a certain entactogenic product. 
My problem is that carbidopa, as well as its derivatives that are also amino acids, is weakly soluble in water and even less soluble in DCM. So it's
hard to do a typical acid-base extraction. If I run a PTC as per Rhodium's article "the methylenation of catechol" and end up with methylenated
carbidopa, I still have to worry about the zwitterionic nature of the intermediate. One patent suggests I could use extract amino acids with a ~1M PTC
catalyst (like Aliquat 336, but I have TBAB) in an organic layer to exchange with a monoanionic form of the amino acid. NaBr / NaI would get left
behind in the aqueous phase and/or precipitate in the organic phase, while a lipophilic dimer of tetrabutylammonium [amino acid anion] would get
extracted into an organic layer. I could then back-extract into acidic water?
[Edited on 16-1-2016 by yanzi] |
I would try to make diiodomethane first from DCM and use it as solvent (bp 181°C - should dissolve it much better and also highly increase reaction
rates) or making water solouble phosphate salt from it or simply use DMF as solvent . - btw. how you do you plan to get rid of the -NH2-Group of
Carbidopa? |
DIM is unstable to light. It's also hard to synthesize from Finkelstein procedure, based on other reports I have read.
Also, I meant methyldopa, not carbidopa.
Why are people so averse to phase transfer catalyst reactions? PTC reactions often proceed with much better yields than single-phase reactions,
especially if the reagent is soluble in one phase and the product is soluble in the other.
|
|
|
yanzi
Harmless

Posts: 16
Registered: 16-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | CH2I2 only reacts with the strongest nucleophiles; if you read Bonthrone and Cornforth's seminal 1964 article on methylenation it explains that the
methylene halides will only react readily with the catecholate dianion (not the monoanion). Amine alkylation won' t be an issue but you may have some
difficulty stabilizing the trianion (since COOH is also deprotonated) in aqueous media. You could (I think?) use NaOEt/EtOH and you won't need any
PTC, but there is a side reaction creating diethoxymethane (an interesting compound, but not the goal).
[Edited on 24-1-2016 by clearly_not_atara] |
One could decarboxylate the methyldopa first by dissolving the methyldopa in cyclohexanol (which I have a litre of) with 1% 2-cyclohexenone (which I
have 100 mL). I have to watch out for the solubility of the methyldopa zwitterion in DCM, so methylenation first might be necessary.
Why do I want to use these other reagents if I have 500 grams of tetrabutylammonium bromide (phase transfer catalyst) sitting in my closet? TBAB is
incredibly cheap -- I bought 500 grams of it from Molport for only 55 dollars. It's useful for so many things, including sodium borohydride reduction.
PTC methylenation is a very different beast from single-phase methylenation, the yields are much higher. I don't get why people are so averse to using
phase transfer catalysts around here, considering how cheap they are?
[Edited on 7-2-2016 by yanzi]
[Edited on 7-2-2016 by yanzi]
|
|
|
Orenousername
Hazard to Self
 
Posts: 79
Registered: 16-4-2016
Location: USA
Member Is Offline
Mood: Regulated
|
|
How do you guys prevent everything from stinking of feces?   
Lol nerds
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
There will always be some smell, but if the reaction is run with proper catalyst and kept at a reasonable temperature (below ~230 C or so) the stinky
byproducts should be reduced to a manageable level. Also make sure to let the mixture cool to ambient temperature or below before disassembling the
setup.
|
|
|
Orenousername
Hazard to Self
 
Posts: 79
Registered: 16-4-2016
Location: USA
Member Is Offline
Mood: Regulated
|
|
Quote: Originally posted by Crowfjord  | | There will always be some smell, but if the reaction is run with proper catalyst and kept at a reasonable temperature (below ~230 C or so) the stinky
byproducts should be reduced to a manageable level. Also make sure to let the mixture cool to ambient temperature or below before disassembling the
setup. |
Well that's somewhat unfortunate. I can deal with the smell and it seems to clean up pretty easy with acetone but my liebig still stinks of it.
Lol nerds
|
|
|
chemgirl
Harmless

Posts: 4
Registered: 22-1-2017
Member Is Offline
Mood: No Mood
|
|
Hello,
I wanted to report my results so far of decarboxylation in mineral oil and ask some advice on workup. I like detail, so sorry:
rxn:
10 g of tryptophan powder was added to 105 mL of light (fischer) mineral oil and 5 mL (R) carvone in a 250 mL round bottom flask with a metal stir bar
and heated with a fiberglass insulated mantle. A claisen adapter was fitted to the flask and a thermometer placed in the center of the oil. In the
other neck a nitrogen line was added and the flask flushed before heating; this was haphazardly done and some oxygen probably got through.
As the temperature increased to 90c the oil was a frothy cream color. At 135 condensation of some sort was seen on the upper layer of the flask (not
hot enough to burn). At 170 the flask got slightly darker. At 200, it was noted that the tryptophan was still present, and the mixture was still
foaming. It took ~30 minutes to get to this point, and the temperature was held for another 30 minutes, never rising above 205. During this time, the
tryptophan disappeared and the mixture got darker. The heat was stopped after the tryptophan was consumed and the flask turned a deep red. After
cooling, the flask turned back to a cream color.
a/b extraction:
After cooling in the fridge, a dark oil settled at the bottom of the flask. the mineral oil above it was tossed and dark oil mixed with a final volume
of 100 mL of 5% boiling acetic acid and filtered into sep funnel. The aqueous layer was like orange juice, and the black goop was extracted in batches
until further addition of acetic acid resulted in a clear aqueous layer.
5 mL chloroform was added and shaked resulting in a clear organic layer with black goop at the boundary between it and the aqueous layer. Organic
layer was tossed and repeated with 2 ml chloroform.
5 mL more chloroform added to aq layer and sodium bicarbonate added until pH was 8 and fizzing was mild. **Only a little bit of stuff crashed out at
this point.** When removed via sep funnel, the chloroform was black. Further washing with chloroform was less black.
2.5g of naoh was dissolved in 10 mL DI water and added resulting in a final ph of 12. The solution turned milky white and and brown stuff came out of
solution. This is probably the goop that was supposed to come out with the bicarbonate.
after:
After chilling in the fridge for a few days, many white fog-like crystals formed above the brown gunk, and was filtered via buchner by simple pouring
(gunk stuck to bottom as cake, soluble in acetone) and washed with cold naoh. These crystals looked white-tan until being squished with filter paper
to aid dryness by me, where they became a yucky tan-brown.
MP was done on some nice looking white crystals (closeup picture attached). Some melting at 103c, a little bit of melting 103-120, and then some brown
goop stayed solid.
So I now have some gross looking mash that is drying, and a bunch of pieces of filter paper that may have absorbed tryptamine via crystals or oil and
I do not wish to throw away. Where to proceed from here? I have many solvents available, so I was thinking re crystallization, but I also wanted to
try sublimation since it is a fun technique.
-ChemGirl

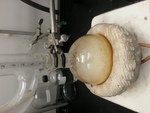




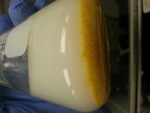




[Edited on 23-1-2017 by chemgirl]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
Hi chemgirl. Nice first post, and welcome to Sciencemadness.
It looks like you managed to get some impure tryptamine. Did you gey any weights of the product?
If your final wash of the crude tryptamine was with sodium hydroxide solution, the product is going to be contaminated with sodium hydroxide and/or
sodium bicarbonate. This is why the final wash is done with ammonia usually. If ammonia is not available, maybe a small amount of ice-cold water
would work.
I always found recrystallization of tryptamine difficult. Feel free to try and report back. Heptane is supposed to work well. If you have access to a
good vacuum pump that pulls a stronger vacuum than an aspirator, I would recommend vacuum distillation through a short path. It's fairly simple and
makes for a reliably pure, crystalline product.
[Edited on 23-1-2017 by Crowfjord]
|
|
|
chemgirl
Harmless

Posts: 4
Registered: 22-1-2017
Member Is Offline
Mood: No Mood
|
|
thanks for your kind words, Crowfjord. The yield in the weigh boat is pretty abysmal, 0.55g. I am salting out the remaining aq solution and am getting
some product back from all the filter paper, but the overall yield will probably be less than a gram. There certainly was plenty of junk produced in
the reaction, and if I were to repeat this I would try harder to get an inert atmosphere.
I will let you know my final yield and what I do for further cleanup.
|
|
|
Racconized
Harmless

Posts: 22
Registered: 27-11-2016
Member Is Offline
Mood: Yes
|
|
Doubt it since (I assume) that it could easily be methylated at the amine probably with alkyl halides or some formaldehyde reaction (I recall seeing
some amine methylation employing formaldehyde but I can't remember the exact procedure)
Also this thread is really old, who bumped
Edit: http://orgsyn.org/demo.aspx?prep=CV3P0723
I don't know if I should post it tbh but it's chemistry afterall
[Edited on 26-1-2017 by Racconized]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Racconized  |
Doubt it since (I assume) that it could easily be methylated at the amine probably with alkyl halides or some formaldehyde reaction (I recall seeing
some amine methylation employing formaldehyde but I can't remember the exact procedure)
Also this thread is really old, who bumped
Edit: http://orgsyn.org/demo.aspx?prep=CV3P0723
I don't know if I should post it tbh but it's chemistry afterall
[Edited on 26-1-2017 by Racconized] |
Bumping? A nice post with a nice write-up you mean I suppose. I guess anyone here prefers a nice write up in a ten year old thread over a
non-reaction with a link to a non-applicable precedure including an assumption without any basis.
[Edited on 26-1-2017 by Tsjerk]
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
20 seconds of google yields -
This,
http://www.sciencemadness.org/talk/viewthread.php?tid=11092
This,
https://www.thevespiary.org/rhodium/Rhodium/Vespiary/talk/in...
and.............
http://chemistry.mdma.ch/hiveboard/tryptamine/000211326.html
/CJ
And this thread is about L-tryptophan decarboxylation, not the methylation of tryptamine.
Nice find all the same, Gordon A. Alles is known as a pioneering legend in the field of phenethylamines..............
[Edited on 26-1-2017 by Corrosive Joeseph]
[Edited on 26-1-2017 by Corrosive Joeseph]
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
alking
Hazard to Others
  
Posts: 252
Registered: 11-3-2016
Member Is Offline
Mood: No Mood
|
|
0.55g from 10g tryptophan is pretty abysmal. I've done this a few times in the past, normally I get a 50%+ yield, generally higher. I've also tried
flushing the system with argon (rather haphazardly, was not totally inert) and putting an air lock on it (a step I see you omitted). With the
airlock/inert atmosphere it nearly eliminated the red oil and I even had crystals forming (within the oil mind you) in the flask after the reaction
cooled. I have unsuccessfully tried to short path distill it resulting in decomposition.
I have recrystallized it from heptane, which is difficult as it only dissolved at a rate of ~1g/100ml and it's difficult to even do that, but it did
result in rather pure crystals. The MP was slightly depressed and still had a slight smell of carvone so it wasn't pure, but another recrystallization
or two should get you to 113C or w/e the proper melting point is, but then 2-3 crystallization is so much work I never bothered.
|
|
|
chemgirl
Harmless

Posts: 4
Registered: 22-1-2017
Member Is Offline
Mood: No Mood
|
|
Yes.
Quote: Originally posted by alking  | | I've done this a few times in the past, normally I get a 50%+ yield, generally higher. I've also tried flushing the system with argon (rather
haphazardly, was not totally inert) and putting an air lock on it (a step I see you omitted). With the airlock/inert atmosphere it nearly eliminated
the red oil and I even had crystals forming (within the oil mind you) in the flask after the reaction cooled. |
I was relying on the stream of nitrogen greatly displacing the oxygen; I see now this was not a good idea. By airlock, do you just mean keep the setup
sealed? Can you elaborate?
...though I am interested in that. I am having trouble finding an actual experimental writeup with the reagents I have. I have read all the googleable
threads on vespiary/rhodium/erowid, which seem to conflict, or uses reagents I don't have (cyano/triacetoxy borohydride). I do have nabh4, formic
acid, and formaldehyde, but the consensus is that ring closure will cause attempts with these reagents to fail. I've heard some reports with zinc
borohydride --- but no real experimental evidence, just conjecture. If anyone wants to give me some advice, I'm all ears.
[Edited on 27-1-2017 by chemgirl]
|
|
|
Crowfjord
Hazard to Others
  
Posts: 390
Registered: 20-1-2013
Location: Pacific Northwest
Member Is Offline
Mood: Ever so slowly crystallizing...
|
|
An airlock is usually a bubbler with mineral oil employed as the liquid, which allows gas to escape the system without letting air back in through
small fluctuations in pressure. They can be in the shape of a sideways S, among other designs. Airlocks are also used by brewers to allow carbon
dioxide to escape the fermenter without allowing air in.
I don't think oxidation would have caused such a low yield. I'm wondering if the large proportion of carvone employed (about 50 mole percent) had
anything to do with it. Some irreversible amine alkylation products may have formed, or some peptide polymer products, due to ineffective stirring. I
have wondered for a while why enone are supposed to be better alpha amino acid decarboxylation catalysts than ketones. Maybe 1,4-addition becomes
more of a problem with high catalyst loading. I would suggest trying again with a much smaller amount of carvone, somewhere around 0.25 to 0.5 mL,
while making sure to stir the mixture well throughout the reaction.
As for methylation, see this thread and the paper that I will post there shortly.
|
|
|
| Pages:
1
..
4
5
6
7 |