| Pages:
1
..
4
5
6
7
8 |
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Well, I grew a moderately sized crystal of CuSO4.5H2O in a jar on the stove (the pilot lights keep spots of the stove warm). Put the heat on the
corner where crude CuSO4 resides, and it moves over to the cold side with the crystal. Of course, the solubility change with temperature is fairly
small, so evaporation is still more important (the jar was not covered).
Tim
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
It sounds strange, but I was able to grow a CuSO4*5H2O crystal by placing a beaker of concentrated solution into a dessicatior loaded with NaOH. All
my other attempts at room temperature evaporation haven't gone all too well with NaCl (tends to effloresce) or MgSO4 (forms needle like crystals far
too readily even with insulation to keep temperature high and cool slowly).
Perhaps alum crystals are easier to grow?
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Quote: Originally posted by chemoleo  | | Same here - I was hugely disappointed with ferrous ammonium sulfate - not only is the colour ugly, but also it's prone to oxidation. But I didn't have
much good experience with FeSO4 hydrate either... the iron curse! |
I know this is a rather old post but how did you attept to make the FeSO4 crystals chemoleo? I boil iron(steel?) in H2SO4 before which left me with a
grey material IIRC which turned green with the addition of H2O. The green solution was filtered and left overnight in a jar where Large FeSO4 crystals
grew all over the bottom with some rather large almost 1 ince in size. If left out oxidation is a huge problem and the "crystals" turn earth tone red.
The solution also began to form brown precipitate until the Green color was completely gone. I think the trick is to start with as concentrated
solution as possible and cap it ASAP and allow it to grow or else it will just form brown shit.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Then, I just grew commercial FeSO4 and AS (reagent grade).
I gave up on these trials - one has to grow them under anaerobic conditions to get large crystals without brown oxides, and cover them with laquer or
something as soon as they face the air.
Oh, and the same with FeSO4 on its own - very pretty while they last but unfortunately not for long! 
[Edited on 22-1-2010 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
FeCl2 is also quite nice -- beautiful bluish to green shade (again, depending on oxidation state), interesting shape (I forget what geometry).
They also tend to melt over time, due to oxidation and hygroscopicity of the resulting product.
Tim
|
|
|
Paddywhacker
Hazard to Others
  
Posts: 478
Registered: 28-2-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Paddywhacker  | There are many chemicals that are only very slightly soluble in water that might grow crystals vie the hydrothermal method. I have been thinking that
Thiel tubes might be useful in this context.
Has anybody experimented with ambient-temperature hydrothermal methods? What apparatus was used? |
There are some cheaper-than-normal Thiele tubes on eBay just now. I have ordered a couple. The sparingly-soluble material could be placed at the
bottom of the elbow sidearm and warmed slightly with a wrapping of resistance wire.
Cupric dihydrogen phosphate might be a good material, or some sparingly-soluble organic cupric salts.
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
Was cleaning out a 2l bottle which had been used to make lead acetate through the CuO/AcOH/Pb process (had a hard lead acetate/copper powder/lead
filing mass at the bottom) and as the hot, filtered wash waters cooled I got this:
http://md1.kapsi.fi/kuvae/lead_acetate1.jpg
http://md1.kapsi.fi/kuvae/lead_acetate2.jpg
The crystal is around 9cm long. 
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Beautiful, yet deadly...
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
A bit of a double post, but, behold:



Ignore the Ru3(CO)12, just a colourful sample I had sitting around. All the others are grown crystals. I had trouble forming a good CoCl2 crystal
because the hydrate was forming first, and eventually the blue CoCl4 2- began crystallizing out (excess Cl- from CoCO3 + HCl) - the vial is only a
small sample, I have a much larger chunk in another jar.
I may try heating the entire mass to dehydration to obtain powdered CoCl2, followed by recrystallizing it slowly from just distilled water. The
crystals are intensely dark, not even a 1000W tungsten light shines through these crystals.
|
|
|
Jor
National Hazard
   
Posts: 950
Registered: 21-11-2007
Member Is Offline
Mood: No Mood
|
|
Very nice. It's a shame FeCl2 is so oxidation sensitive, you can see some brown colors on the green crystals, the of oxidation by oxygen.
The cobalt chloride is indeed very dark. I have 250g of small crystals, these are intense red, but not nearly that dark!
How did you make the Ru3(CO)10, or is it a commercial sample?
[Edited on 7-4-2010 by Jor]
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Its a commercial sample from a friend, I originally took the picture to show off some more colourful samples that I had.
As for the FeCl2, I have a bunch of larger crystals that I ended up washing off with DI water, toweling off, and then finally storing under mineral
oil. This small vial of FeCl2 has stored fairly well actually, but indeed it is extremely oxidation sensitive. On the bright side, I did prepare a
large amount of FeCl2 without inert gas (ie: using vacuum and water vapour to minimize oxygen, and working very quickly).
I also suspect that part of the reason why the crystals are so dark is because its a mix of CoCl2 and CoCl4 -2, with overlapping absorption spectra.
Chances are I'll just drive off all the extra water and HCl by heating under vacuum one day, and redissolving/crystallizing.
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
I did get intensely dark crystals when preparing CoCl2 hydrate. It's simply a matter of the bulk, as far as I can tell. Crushed coarsely they assume
the gorgeous deep garnet shade I'm more used to (I also rinsed with a bit of water, but that's not really important for the color, IIRC.
[Edited on 4-7-10 by UnintentionalChaos]
Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
Panache
International Hazard
    
Posts: 1290
Registered: 18-10-2007
Member Is Offline
Mood: Instead of being my deliverance, she had a resemblance to a Kat named Frankenstein
|
|
Quote: Originally posted by aonomus  | It sounds strange, but I was able to grow a CuSO4*5H2O crystal by placing a beaker of concentrated solution into a dessicatior loaded with NaOH. All
my other attempts at room temperature evaporation haven't gone all too well with NaCl (tends to effloresce) or MgSO4 (forms needle like crystals far
too readily even with insulation to keep temperature high and cool slowly).
Perhaps alum crystals are easier to grow? |
Sodium chloride crystallizes excellently by boiling a saturated solution and then slowly adding NaOH, to about 5-10% of your volume ensuring the
addition is slow enough not to crash out any NaCl, this interestingly causes the solution's bp to increase and artificially creates a super saturated
brine solution. Let this solution cool and stand for some time (about a week, covered to exclude dust), sodium chloride crystallizes out in a very
spectacular fashion. I often give these as gifts to young relatives (after removing them from the caustic solution and rinsing in dry alcohol) saying
i grew them myself. They are hardy enough to gently handle but fragile in their cubic detail, they seem like small cubic cityscapes. I'll hunt for a
photo or maybe i'll just grow another.
|
|
|
aonomus
Hazard to Others
  
Posts: 361
Registered: 18-10-2009
Location: Toronto, Canada
Member Is Offline
Mood: Refluxing
|
|
Thats interesting that 5-10% NaOH causes the NaCl to saturate. I'll have to try that this week.
I'm also in the process of building a crystallization chamber based on the rotating platform setup the LNL used to grow massive KDP crystals for their
big lasers.
http://education.llnl.gov/crystals/Sketch.html
[Edited on 25-7-2010 by aonomus]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Holden and Singer — Crystals and Crystal Growing
I have not read the entire thread, however, I didn't
find this when I searched for it.
Alan Holden and Phylis Singer
Crystals and Crystal Growing
Anchor Books 1960
I have always found the book useful. You can buy a used copy
for a buck.
Byda - I have only used this to grow KNO3 xts. Take a hot saturated solution of
whatever - and put it in the freezer.
Saywhateverhappened to rock candy crystallized sugar?
http://en.wikipedia.org/wiki/Rock_candy
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Prismatic Copper Sulfate
Observed something interesting this evening.
Solution: an unknown mixture of CuSO4, NaCl and etc. In particular, I think the chloride concentration is low enough that
CuCl4<SUP>2-</SUP> is not forming in large concentration. The solution is bluish green, not bright green. Hence, the copper is
crystallizing as CuSO4, while a little NaCl (if that's what it is) crystallizes as colorless crystals. The remainder will probably eventually produce
the neon-green Na2CuCl4, possibly as an intederminate mixture with NaCl and CuSO4.
Method: solution was placed in a drying tray and placed over a warm spot. A gas stove was used, with pilot lights below the working surface which
heat it in spots to about 90C. This caused two convienient hot spots on the tray, where little to no CuSO4 formed, but NaCl formed easily. This
suggests little change in solubility for the NaCl, which is consistent with its known properties.
Photos: these show the crystals, which seem to be prismatic, and monoclinic I think. A dramatic change from the sharp, hard rhombic habit of pure
CuSO4. These crystals were rather weak and easy to crush, though they adhered to the tray quite well.
The tray is yellow plastic, so most of the background also looks yellow. The yellowness is enhanced by the residual supernatant, which was viscous
and drained poorly. White streaks glint from its surface, while around the blue crystals, a green halo emphasizes the color of the solution.
Tim
 
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
I too have noticed the different habits of copper sulfate- It actually switches between a few- Your normal prismatic, then the crystals grow flat,
then a much more cubic prismatic (I can provide pics, if you'd like) which is a lighter shade of blue, then monoclinic.
Also of note- I've discovered that the monoclinic crystals can be melted and used as inlay  I have no testament to the longevity of this procedeure, though! I have no testament to the longevity of this procedeure, though!
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Curious, I wonder if this is a heptahydrate analogous to NiSO4, FeSO4, MgSO4, etc. Cu ions don't normally fit into this crystal.
Tim
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
I am trying to grow some moderately sized sucrose crystals , to experiment their rumoured chemiluminescence effect on breaking.
I dissoved 350 g sucrose in 100 ml of water, boiled it, it all dissolved, then left it with a lid, and insulated with blankets . After a day it
cooled, but all the sugar is still in solution, even though its solubility at 20C is 200g/100ml H2O. The liquid is extremely viscous, much more than
glycerol.
What should I do? Should I boil it again to get rid of a little more water, and try again?
Or would the addition of a little NaCl have an effect of the solubility of sucrose, like in the 'salting out' of soap?
thanks
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Try adding a few grains of sucrose. It will eventually crystallize (thermo says so), but it could take weeks, or "forever".
Syrups are annoying things. I do know this: we've never had a jar of honey in the house which did not eventually crystallize. Temperature cycling
over months-years seems to help.
I would suppose "crystal candy" either starts with a roughened stick (thus providing a few random points that are likely to have something shaped like
a sugar molecule to fit into and thus grow from), or I suppose they could roll it in sugar first, to provide actual crystals.
Tim
[Edited on 9-13-2010 by 12AX7]
|
|
|
Wizzard
Hazard to Others
  
Posts: 337
Registered: 22-3-2010
Member Is Offline
Mood: No Mood
|
|
What you need to do is keep adding sugar into boiling water (or at near bioling) untill NONE will dissolve, then put the beaker/jar into a great
thermal ballast, allowing it to cool VERY slowly over a few days. That'll do the trick- I got about 1cm talkl sugar house-shaped crystals 
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Just in case someone wonders what a room looks like that has been filled with a hot solution of CuSO4 after cooling:
http://www.guardian.co.uk/artanddesign/2008/sep/04/art

I think I may try a project like that one day! 
But obtaining 80,000 L of sat. CuSO4 could be tricky!
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
I was wondering who bought all of those. 
I hear they have a high resale value as esoteric walking canes among the gentlemanly garden gnome community.
[Edited on 24-1-2011 by peach]
|
|
|
Endo
Hazard to Others
  
Posts: 124
Registered: 5-1-2006
Location: USA
Member Is Offline
Mood: Cold
|
|
Crystal Growing
Having about a week before my 9 yr old daughter is due to have her thrid grade science project finished. I pointed her to the lists of subjects easily
found online until she found something that hit her fancy. She decided that she wanted to grow crystals, and setup as her tentative hypothesis: *That
if she mixes two different solutions she will get a mixed crystal that doesn't look like what is grown from either solution.*
So my goal is to be able to have a few crystals of two of the substances that she can show. Then show a crystal grown from a mixture of two
solutions. (understanding that the crystal that forms first will be the compound with the lowest solubility)
Having a lot of chemistry stuff laying around I decided that this was doable. Having the previously mentioned crystal growing webpage on my bookmarks
I attempted to setup the evaporation method using three different solutions, Copper sulfate penta hydrate (I had five pounds laying around), Magnesium
sulfate (also laying around) and Potasium Aluminum Sulfate(Alum) also laying around.
I find that with the weather running like it is Varying between -30F and +30F with the heat kicking off and on a lot; that the suspended seed crystals
would dissolve/partially disolve and fall to the bottom. Getting frustrated with the problems I decided to setup something with a controlled heat
system. Scavenging an old crockpot destined for goodwill, and a temperature controller salvaged from a parts hotplate stirrer I wired it in and with
about 4-5 inches of vegetable oil in the crockpot I can now easily control the temp from ~200F on down.
So my questions:
When making a concentrated alum solution I can't seem to get it to clarify. It ends up with a milky look to it which doesn't settle out on sitting.
I have tried boiling the solution and I am using distilled H2O. Filtering doesn't seem to help. I suspect that this milkyness to the solution is why
when maintining the concentrated solution over the last four days, decreasing the temp ~5F every twelve hours, my alum solution filled my
crystalization jar full of crystals. (I am assuming that the suspension acted as a large nubber of seeds).
My Copper sulphate crystalization jar ended up with a sheet of copper sulphate crystal across the bottom. I assume my seed dropped to the bottom and
because I didn't check it filled up the bottom of the jar.
The Magnesium sulfate solution ended up super-saturated quickly. When I disturbed it to try to introduce a new seed it turned to slush.
So last night I started over, bringing each solution up in temp until nothing else would dissolve, (yeilding a milky alum solution again) and brought
the temp back up, starting at 175F this time.
I am wondering if I can get good growth from these solutions by adding more oil, bringing it up to temp, and allowing the oil to cool slowly
overnight.
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
With the mixed crystal idea, recrystalisation is used specifically to purify one salt from the other, as the salts tend to want to solidify on
a lattice that is of their own kind.*
This page is written by someone else trying exactly the same Alum experiment and ending up with opaque solutions
You may be right with regards to the seed crystal hitting the bottom in the others, and other crystallisation points appearing.
With a week to go, time is of the essence! 
I would offer the possibility of an entirely different approach. The salts you are using, like copper sulphate, loose their pretty colours when in
their anhydrous form (when all of the water of crystallisation is driven off).
You can achieve this by simply roasting the more stable salts, like copper sulphate, in the open air. The water content goes through specific stages
at different temperatures, as discussed in the "chemical properties" section of the Wiki article.
As soon as any water gets near the powder, it will go back to the beautiful blue.
This is a very visual demonstration and one that can be done quickly if she'd like to do it in front of the teacher or class.
There is also a wide selection of the level of detail you can go into as to what is happening. The water is changing the colour by forming ligand
coordination complexes with the salt, which affect the orbits of the electrons and how they adsorb light; the colour they show is the colour they are
not adsorbing.
After googling what 9th grade is in the US (as I'm from the UK), I think it would suffice to say, it's making the salt molecules group together and
share electrons in a different way. Paint pigments are based on transition metal complexes as well.
If you scroll down this Wiki article to "Pigments by chemical composition", you can see there are many of them based on copper; Azurite, Han purple,
Egyptian blue, Malachite, Paris green, Phthalocyanine Blue BN, Phthalocyanine Green G, verdigris & viridian. But they don't rely on water of
crystallisation to maintain their colour - a noteworthy difference. Synthetic pigments where high tech stuff in ancient Egypt and Rome, and brightly
coloured paintings, objects and clothes where a sign of being rich.
Today's equivalent are quantum dots, which absorb one band of light and emit a very specific, narrow band of another - costing hundred to thousands of
dollars per bottle.
Another point that ties in with this is plants, as the petals use metal ion complexes to produce the bright colours needed to attract bees - the
brightness is affected by soil pH (with bright flowers usually wanting more acidic pH's). And the chlorophyll that sustains life on Earth contains a
Magnesium complex.
There are also different things she can say about the two different forms of hydration in her experiment. Anhydrous salts, due to them not being
crystalline, can be easily ground to extremely fine powders, whereas those with water of crystallisation tend to be big lumps (if you can buy a decent
sized crystal from eBay, she could use that as an example of what the anhydrous salt won't easily do).
She can also use the property to demonstrate a use. If the salt is added to a none polar, dry solvent, nothing will happen. If there is moisture in
the solvent, the powder will begin going back to it's more well known colour. So it can be used to detect the presence of water. She could use colour
changing silica gel, used to dry flowers, as an example of where that's useful in everyday life and how it's used (by baking it in the oven to drive
the moisture off again).
She can liken the process to a container of table salt 'going funny' if left in a damp cupboard (particularly if you have one in your cupboard). As
well as how lumps form in powders and how this can clog up machinery that processes them.
The only safety problems are a.) don't eat it (which applies for growing crystals) b.) when you grind up anhydrous salts, they will tend to float
around in the air, even if you're doing it gently in a mortar and pestle.
The salt WILL end up up your nose and with something like copper sulphate, it WILL sting. You'll be able to taste copper coins in your mouth almost
instantly, which is nice at first, but starts to get annoying and burns because it lingers for hours inside your nose. A dust mask is a good idea.
Transition metal salts will work better for the demonstration because you'll get bright colours. For obvious reasons, you want to avoid the more toxic
options; like the chromates. Some salts, like ferric chloride, will also decompose if heated in the open atmosphere, releasing hydrogen chloride.
Copper sulphate is a safe bet, and they likely use it in her classes anyway.
The salt will heat up as it's rehydrated. Which provides another example of how the process is reversing; heat is needed to drive the water off, and
then heat is given back out as the water is put back.
This may seem somewhat boring, but then, crystal growing is the de facto standard for a lot of science projects it seems. With this example, whilst
you don't get big crystals (which no one has seen you grow anyway, so they could be off eBay), you see it happening immediately, it advances on the
growing by demonstrating the different properties and seems more unique to me.
I would imagine a chemistry teacher would be more impressed by linking all the properties and uses together, as opposed to seeing another crystal.
To show off, she could say how testing for water is extremely important in some chemistry experiments. In university labs, they will use something
like the Karl Fischer method; which doesn't work in the same way, but it's testing for the same thing.
If you put a sample of the freshly baked salt on a cheap pocket scale at the beginning of the demonstration, you will likely be able to see that it
has gotten heavier by the end, despite not being pure blue again, as it picks moisture up from the atmosphere.
"This is one of the reasons why chemicals must have their lids put back on straight away" <---- every chemistry teacher is going to like hearing
this.
Then call up Aldrich and ask them to send you an empty Sure Seal bottom by express delivery for urgent science project work. 
Worth a try! The worst they'll say is no.
NurdRage has an Alrich account, and he's lurking around on here. Maybe you can harass him into scoring you an empty bottle. 
Here's a page all about the process
If you follow this example, using a test tube for the drying, you can show the water leaving. I've dried quite a few salts by emptying
them out onto the hotplate and roasting them that way, but the water will come off as steam, which isn't as graphic as droplets. It can also get messy
(as it splatters) and kids could burn themselves doing it.
Water on anhydrous copper sulphate - need to be careful you don't get any fine powder up your nose as the water goes on

A 3,000 year old blue pigment found in Egypt

Chlorophyll A
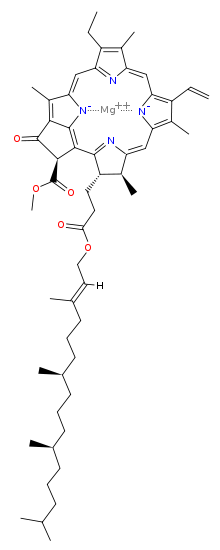
[Edited on 4-2-2011 by peach]
|
|
|
| Pages:
1
..
4
5
6
7
8 |