| Pages:
1
..
4
5
6
7
8
..
13 |
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
hector, that's a very interesting picture. Is there a schematic drawing and/or flow chart of that pilot plant as well?
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
This is real plan.and this plan use new method for making tnt(new part in washing step)


[Edited on 18-3-2008 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
What is meant by ONT? It would appear to be the Toluene feed stock?
|
|
|
microcosmicus
Hazard to Others
  
Posts: 287
Registered: 31-12-2007
Member Is Offline
Mood: spin up
|
|
I assume he is being cute and writing "oudeno-nitro-touluene (0NT)" in
analogy with "mono-nitro-toluene (MNT)", "di-nitro-toluene (DNT)", and
"tri-nitro-toluene (TNT)". "Ouden" is the Greek word for "nothing" ---
actually "zero" is "meden" in Greek, but since that starts with an m
we can change the word a little.
[Edited on 19-3-2008 by microcosmicus]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I'm sure he means ortho- nitrotoluene.
Since the "mononitrotoluene" from the "first step" in TNT synthesis contains a few percent m-NT, the resulting TNT contains 3,5-DNT and is too impure
for lots of applications.
By using pure ortho-NT, which is an important chemical intermediate for other things (toluidine, dyes), as the precursor this problem is circumvented.
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
ONT=ortho nitrotoluene

the step of toluene Nitration
http://i27.tinypic.com/200332u.jpg
old sulphite washing step:
http://i29.tinypic.com/6izi1y.jpg
[Edited on 19-3-2008 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Thanks, I understand the reason for using ONT as the initial feed now- From the perspective of someone proceeding with over the counter chemicals, it
would probably be easier to start with toluene and do a sulphite wash of the crude product?
[Edited on by Bert]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
Ortho-NT can be obtained from mononitrated toluene by fractional distillation, it boils at 222°C while p-NT boils at 238°C.
The p-NT is preferrably first separated from the crude MNT by freezing out with ice and salt, the separation is not entirely complete however, hence
the need for subsequent fractional distillation.
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
Yes Sulphite Waqshing is Necessary If you want to have Pure TNT.
Anyhow (with ont or pnt or mnt) in the final Product(tnt) you will have other impure like c(no2)4 and... then sulphite washing or crystalization is
necessary.
Chemistry=Chem+ is+ Try
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
Today I tried to make MNT. To 170 ml of H2SO4 (boiled down battery acid from a old car batter, very contaminated y some needle crystals, probably lead
sulfate) I added 85 g of NH4NO3. When the ammonium nitrate dissolved I added 90 ml of toluene to the mixture. The toluene turned to clear yellow and
then I heated the mixture to start the reaction Then the reaction started and I overheated it a bit so I coled it down in a water bath. Then I stirred
the mixture with a measuring cylinder for around 20-35 minutes. After that I let it to sit and cool for around 45 min to 1 hour. Then I diluted the
mixture with around three times of its volume with water and decanted the top layer (it turned from red to non clear orange). Then I added the MNT to
water. And the MNT floated on top. It is supposed to sink (it always sank). And I could smell toluene in it. So it looks like it isn't completely
nitrated. Does anyone know what did I do wrong?
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
I have tried to remove the leftover toluene (and a small amount of water) from the MNT by boiling it away on a low gas flame. At first it didn't do
anything, then suddenly splashed a few times, then for a minute or two it boiled nicely and then suddenly again it splashed forcefully (a lot of it
went out of the baker). Some landed in the burner flame so everything caught on fire. I removed the baker from the burner and closed it with a flask
to extinguish the fire. But the remaining MNT is now contaminated with fine carbon dust At leas it now sinks in the water. But I think that there is still some toluene left in it. Does anyone know why it splashed like that? At leas it now sinks in the water. But I think that there is still some toluene left in it. Does anyone know why it splashed like that?
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
You said it had some water left in it. Water boiler at a lower temperature than toluene or nitrotoluenes, and the water-toluene azeotrope is lower
boiling yet. Those conditions make it easy to superheat the water, which can lead to explosive boiling. You didn't describe your workup of the
reaction products, you may have also have had dissolved salts and/or acid in the water; with much acid+nitrate you could have started nitrating the
mix again.
It's always a good idea to dry liquids with low mutual solubilities with water before attempting to boil said liquids.
It's a bad idea to heat, especially boil, flammable substances over an open flame. It's even a worse idea to do this with nitrated organics. It's
especially bad to do so when there's still droplets (or more) of water in them.
Get yourself an electric hotplate, and/or get a simple distilling rig, and use a sandbath to heat the flask with the toluene/MNT in it. Rig thongs so
toluene vapours don't drift onto the heater.
|
|
|
MagicJigPipe
International Hazard
    
Posts: 1554
Registered: 19-9-2007
Location: USA
Member Is Offline
Mood: Suspicious
|
|
I figured it would be common sense to heat any flammable liquid with a flame. It just seems very counterintuitive.
Think about it. If the vapors collect somewhere and then somehow come in contact with the flame (or superheating occurs while oxygen is present in
the reaction flask) you could have a fuel-air explosion on your hands. And don't say that's impossible because it's not. It just seems to me that it
would just make sense not to heat flammable liquids with flame.
Would you boil gasoline on a gas kitchen stove? Even if you thought the reaction vessel was closed?
Sorry Zinc but this time I have to say, "DURRRRR!!!!!".
"There must be no barriers to freedom of inquiry ... There is no place for dogma in science. The scientist is free, and must be free to ask any
question, to doubt any assertion, to seek for any evidence, to correct any errors. ... We know that the only way to avoid error is to detect it and
that the only way to detect it is to be free to inquire. And we know that as long as men are free to ask what they must, free to say what they think,
free to think what they will, freedom can never be lost, and science can never regress." -J. Robert Oppenheimer
|
|
|
Zinc
Hazard to Others
  
Posts: 472
Registered: 10-5-2006
Member Is Offline
Mood: No Mood
|
|
Ye I know it was a stupid idea to heat it with a gas burner. But I don't have a hot plate. I must get one.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Zinc
Ye I know it was a stupid idea to heat it with a gas burner. But I don't have a hot plate. I must get one. |
"Yes, it was not wise to step off the top of the cliff, but there wasn't a bridge across the canyon so I went ahead"
"True, the ship was unfinished and had no bottom, but I was in a hurry to set sail"
Sometimes it s better to not start the voyage when something critical is missing.
|
|
|
Axt
National Hazard
   
Posts: 861
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
<b>Preparation of explosive substances in the laboratory. II. Preparation of trinitrotoluene</b>. Tseng, Chao-Lun; Tuan, Kun.
J. Chem. Eng. (China) (1937), 4 59-64. Journal language unavailable. CAN 31:31940 AN 1937:31940 CAPLUS
Abstract
cf. C. A. 30, 2003.3. Convenient lab. procedures for the prepn. of TNT (I) are described. One mol. PhMe, 132 g. concd. HNO3 and 190 g. concd. H2SO4
give at 30° 134.5 g. MNT (II) of d2525 1.166. One-half mol. of II, 49 g. HNO3 (sp. gr. 1.52) and 98 g. concd. H2SO4 give 86 g. DNT (III), the
reactants being mixed at 60-70° and later raised to 90-100°. 36.5 g. of III, 146 g. concd. H2SO4 and 36.5 g. HNO3 (sp. gr. 1.52) give 43.5 g. of I;
during this prepn. II is first dissolved in H2SO4 with warming, then HNO3 is added at 60°, and the temp. is subsequently raised first to 95° and
finally to 120-5°.
[Edited on 24-5-2008 by Axt]
|
|
|
Leander
Harmless

Posts: 28
Registered: 23-2-2008
Member Is Offline
Mood: No Mood
|
|
Last week I tried to produce some TNT myself, which resulted in a really strange product.
The synthesis was done in two steps, using the method Sobrero mentioned earlier in this topic to go strait from toluene to DNT using H2SO4 and a
nitrate salt @ 60oC. This method gave me a 92% yield based on toluene, of slightly yellowish dinitrotoluene.
To add the last nitrogroup, I decided to use H2SO4/NH4NO3 again. A 75/25 H2SO4/HNO3 nitration mixture by volume was made, forgetting the NH4HSO4. The
water content of the AN was onknown. The DNT was added in a 1:2 molar ratio of DNT/HNO3. After 2 hours of nitration @ 95-100oC with occasional
stirring the acid was quenched in ~600 ml of water. Instantly a yellowish oil sunk to the bottom. The oil was washed with strait water and dried for
further examination. Probably the relatively high water content of the mixture was the cause of the byproducts forming.
The crude oil seems to have a melting point of 5-8oC, and a density of 1.35 g/cc. After a day or two I noticed the colour of the liquid became
slightly more orange, so I decided to wash the product with 5% ammonia solution. Upon contact with the oil the ammonia graduately turned bloodred.
After washing with water again, the now yellow oil suddenly became a lot thicker, to an almost solid state. The stuff deflagerates from flame really
lazy, taking over 10 seconds to fully decompose for a ~.5 ml drop, smelling really.. uuhhm.. 'nitro'! 
Does anybody has any idea what the heck this product is? Since the nitration was done on a relatively moderate temperature with graduate temperature
changes, I assume the benzene molecule is still intact. I assume most of the mixture consist of tetranitromethane, since it has a melting point of
~13oC, and is also a liquid at room temperature. Also, it's is a well known impurity in any TNT synthesis.
[Edited on 4-8-2008 by Leander]
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
Patent http://www.pat2pdf.org/patents/pat6881871.pdf has this to say about TNT manufacture:
| Quote: | Current methods of synthesizing TNT involve successive mixed-acid nitrations of the petrochemical toluene
(Milligan, B., "Isomer Distribution in Mixed-Acid Nitration
of Toluene. Evidence for Mass-Transfer Efects on Selec
tivity" Ind Eng Chem Fundam, 25(4), 783-789(1986). During this process, some undesired nitration isomers occur (a-, beta-, and
y-trinitrotoluene) as well as incomplete nitration products (2,4- and 2,6-dinitrotoluene). These unwanted isomers are removed by sellite (sodium
sulfte) washings.
Isomers with nitro groups ortho to one another react with the sulfite generating ionic, water-soluble products. These
washes have a characteristic red color, hence the name `red
water'. These washings are highly toxic and very expensive to destroy (incinerate). Because of this `red water'
problem, there are no North American plants that will produce TNT.
Currently, all U.S. needs for TNT are supplied from overseas
sources where environmental standards are mild or non
existent.
|
This patent offers an alternative (but elaborate & expensive) synthetic alternative to classic toluene nitration:
[Edited on 5-8-2008 by Ritter]
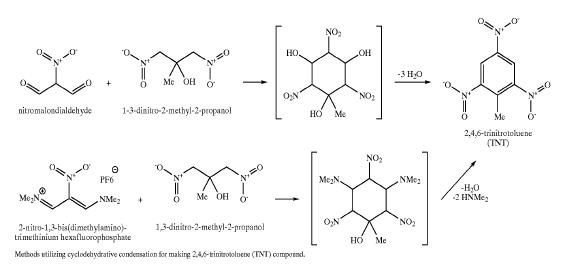
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
My answer to this topic:
http://www.sciencemadness.org/talk/viewthread.php?tid=11269
Making Tnt isnt Difficult!!
Mononitrotoluene: use 2 mol nitric acid(65%) for every mole Toluen(better you extract ont) and 4 mole sulfuric acid(96%-98%)
Dinitrotoluene:use 2 mole nitric acid(96%-98%) for every mole Mononitrotoluene(ont is better) and 4 mole sulfuric acid(96%-98%)
Trinitrotoluene:use 2 mole nitric acid(96%-98%) for every mole Dinitrotoluene and 1 mole Sulfurtrioxide(dissolve in sulfuric acid)(oleum 20%)
about convert dnt to tnt:
Heat solution 3 hour at 110-115
Picture of final step(dnt to tnt):
Left beaker: oleum(20%)+nitric acid 96%
Right beaker: Dnt+sulfuric acid







Final Product melting point(without sulphite washing):75c
[Edited on 6-10-2008 by hector2000]
Chemistry=Chem+ is+ Try
|
|
|
Cyrex
Harmless

Posts: 12
Registered: 2-10-2008
Member Is Offline
Mood: No Mood
|
|
thanks @hector
i made trinitro according your recipe and my product melting point is 75 but when i add sodium sulphite(5 time it weigh 5%) my product color will be
red and after washing it color wont change
my product is 500gram and after washing with 100lit water it decrease to 300gram and its color change to light brown
really i made tnt?
how can i pure it?
|
|
|
hector2000
Hazard to Others
  
Posts: 127
Registered: 22-8-2006
Member Is Offline
Mood: Cool
|
|
Mr Cyrex Please Explain Your Washing Step
Yes.really you made tnt.
if you use ont(ortho nitro toluene)then you wont have red tnt and there is no need for sulphite washing.
Pure Tnt Pic:

Chemistry=Chem+ is+ Try
|
|
|
jmneissa
Harmless

Posts: 29
Registered: 4-5-2009
Member Is Offline
Mood: No Mood
|
|
hey so sorry to reopen this old thread but this is by far away the best thread on TNT on these forums. Now my question is that there is a lot of
process out there for making TNT. I have come to the conclusion that the 3 stage process is the best one. Now after reading books on TNT synthesis I
have read about the so named "German Process" that is in the first stage the Toluene is reacted with nitric acid alone to reduce the formation of DNT
and TNT. From this the ONT is extracted for use in TNT manufacturing. After this it is a simple two stage process. Reacting the ONT to DNT and the DNT
to TNT. The question I have are the ratios of acids to other acids and the total ratio of acids to toluene per each nitration. Also I have a question
about hector2000 picture. If he is using ONT as a starting material why does he have three nitrators? He only needs two. I see that some people have
been more successful with different methods. I have no clue which method is better I have seen different concentrations of acids different
temperatures, heating, cooling, NO2 fumes, no NO2 fumes etc..... There is such a broad scale of what will work. I was hoping someone would be able to
point me int eh right direction a certain synthesis which is know to produce a high yield.
For Example in one synthesis I have read it says to just add all the toluene straight to the acids without regard for temperature. On the other hand
another synthesis says carefully monitor temperature and not allow to go above a certain point and add acids to toluene not the other way around.
Which way is right?? I don't know... This is continued throughout the broad range of synthesis I have read some separate spent acids others keep
them... If anyone can clear up this I would greatly appreciate it! Thanks a bunch
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
There are several effective methods of nitration depending upon the needs of the surround. In plant level nitration, the needs of both refinement of
the final product & the re-cycling of the acid have great impact. In a lab demonstrating levels of nitration as an education instrument; the needs
are different. There really is no "best method". I have even seen crystal growth procedures, yielding 2.5 cm needles & the method of nitration(s)
were designed for that agenda! TNT used to made in hundreds of Organic Chem classes exactly for that reason. It's damn easy to do, the issue of
toxicity aside.
Frankly I would always monitor the temp level or the efficiency of your lab is compromised by oxidation; amongst other issues. Somewhere is the
posting of the needles but the pic is not as sharp as could be. The sodium sulfite wash is a good idea as it is said to prevent the formation of
"oils" in the final product. Depending upon the ability to monitor temp a promising mp is close to 80C; lower than that and there would exist a larger
% of DNT in the final product. In terms of dangers there exists some if the temperature climbs as the manipulation of a flammable vapor exists
initially on the surface of the reaction vessel.
[Just an opinion but] simply from the standpoint of one's notes, if the temperature is not recorded, the reaction from one level to another becomes
sloppy & more akin to a "lucky-recipe" than anything else.
[Edited on 1-6-2009 by quicksilver]
|
|
|
darel
Harmless

Posts: 27
Registered: 12-7-2008
Member Is Offline
Mood: No Mood
|
|
I have Jared Ledgards Third Edition of the Preperatory Manual of Explosives. In it, he gives two one step nitration synthesis.
The First:
TNT can be made by treating toluene with 99% nitric acid in the presence of premium unleaded Gasoline. The TNT is recovered by recrystaliztion and
purified with 70% sulfuric acid.
The Second:
TNT can be readily prepared in a one step process using Boron trifluoride as a catalyst. 95-99% nitric acid is refluxed with toluene and BF3 at 90c
for about three hours put into a seperatory funnel and the top half is TNT. The bottom fraction containing the BF3 is recycled. The TNT is purified by
refluxing with 98% sulfuric acid for one hour.
Has anyone ever played with this. I found Boron for $68 a lb. Mix with HF to get BF3.
I will try this as soon as I get The BF3.
http://books.google.com/books?id=J55D3HcgPuoC&pg=PA276&a...
Page 276 for the BF3 catalyzed and bottom of 271 is the beggining of the Gasoline route.
[Edited on 8-1-2009 by darel]
|
|
|
entropy51
Gone, but not forgotten
    
Posts: 1612
Registered: 30-5-2009
Member Is Offline
Mood: Fissile
|
|
darel, I don't know enough about TNT to comment on these specific preps, but be very careful of anything in any of Jared Ledgard's little books.
Some of what he writes is demonstrably horse hockey, some of it dangerous.
|
|
|
| Pages:
1
..
4
5
6
7
8
..
13 |