| Pages:
1
..
4
5
6
7
8
..
77 |
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
The extraction was done a few months ago so I'll have to check my notes and get back to you. It was basically just precipitating the gold out of aqua
regia with sodium metabisulfate which gave gold powder. Then the powder was melted down with two MAPP gas torches. I'm sure the gold is quite pure
just based on the composition of 14k gold... Nothing else should have precipitated out.
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|


That awesome moment when a reaction overreacts. It climbs out from the flask, directly to the wall of the fume hood.
The sad part: it was a concentrated sodium methoxide solution what will be great to clean up. Organic chemistry <3
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Strontium nitrate/potassium perchlorate, and aluminum flash powder:

Intentionally slow burning, (so I could get it on camera).
[Edited on 21-1-2014 by Zyklonb]
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
kristofvagyok, I love that pic of you flicking off that horrible mess. It shows the (sometimes) shitty side of chemistry.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Yes, I think we've all been there.
Kristo could you tell us the nature of the reaction, and the scale?
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
kristofvagyok
National Hazard
   
Posts: 659
Registered: 6-4-2012
Location: Europe
Member Is Offline
Mood: No Mood
|
|
It was an intermediate during the synthesis of cubane, according to this, the third step:
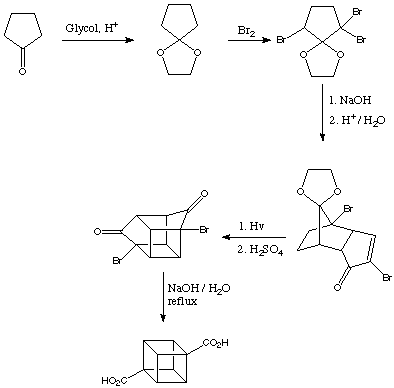
The scale was a circa 400g.
A picture from the bromination:

[Edited on 21-1-2014 by kristofvagyok]
I have a blog where I post my pictures from my work: http://labphoto.tumblr.com/
-Pictures from chemistry, check it out(:
"You can’t become a chemist and expect to live forever." |
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
And I suppose it was the final step. My sympathies.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Quote: Originally posted by Bot0nist  | | kristofvagyok, I love that pic of you flicking off that horrible mess. It shows the (sometimes) shitty side of chemistry. |
Although I have heard it both ways, I think the correct verb, to give the finger, is flipping off, not flicking off.
Some people say that you flip off a person, and you flick off a thing. E.g. mess. I don't know if that's true though.
|
|
|
ElizabethGreene
Hazard to Others
  
Posts: 141
Registered: 15-10-2012
Member Is Offline
Mood: No Mood
|
|
The CD V-715 is an Ion chamber gamma ray detector used to detect (large) amounts of radiation subsequent to a nuclear war. It was manufactured and
distributed through the US Civil Defense program in the 1960s. The "little brother" of this device is a Geiger counter. Those are built around the
geiger-muller tube in the second picture. The two devices work on different mechanisms to do the same job.
The range of this detector is measured in roentgens per hour. The normal radiation dose for a person is measured in hundreds of milli-roentgens per
year.
Terrifyingly, there were 0-50 R/hr and 0-500 R/hr meters too. I'd rather not ever see that needle move off zero, thanks.
Bfesser: That meter is in fantastic shape. Did you restore it?
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
quoted images]
[Edited on 24.1.14 by bfesser]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Here's some tetraamminecopper(II) sulfate and tetrachlorocupric acid (is that what you'd call the green product formed by HCl + CuCl2?).

A cool demonstration to do is to heat a vial with iodine until it vaporizes. Cap the vial (being careful not to burn yourself in the process) and
invert it. The result is an iodine cap over the vial, like this (it didn't form completely, and the photo's a little blurry):

At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
bfesser
Resident Wikipedian
    
Posts: 2114
Registered: 29-1-2008
Member Is Offline
Mood: No Mood
|
|
Surprisingly, no. It was in this condition
when I purchased it from the surplus shop. The only problem I found was that the ferrite core of an inductor had broken loose and was sliding freely
back-and-forth through the bobbin, when tilted. I 'fixed' this with a small piece of clear Scotch tape over each end. With a D cell installed, the
circuit check and zero work as expected; as far as I can tell, it's in perfect working order—I don't have a γ source strong enough
to deflect the needle.
  <a href="http://www.flickr.com/photos/35937732@N02/11920075066/" title="CD V-742 by bfesser, on Flickr" target="_blank"><img
src="http://farm4.staticflickr.com/3832/11920075066_be60878034_n.jpg" width="200" height="150" alt="CD V-742"></a> <img
src="../scipics/_ext.png" valign="top" /> <a href="http://www.flickr.com/photos/35937732@N02/11920075066/" title="CD V-742 by bfesser, on Flickr" target="_blank"><img
src="http://farm4.staticflickr.com/3832/11920075066_be60878034_n.jpg" width="200" height="150" alt="CD V-742"></a> <img
src="../scipics/_ext.png" valign="top" />
As for the range, let's just say that I'd rather not have need to use this meter, nor would I like to see deflection in the filaments of my 200
Röntgen dosimeters.
[Edited on 30.1.14 by bfesser]
|
|
|
zenosx
Hazard to Others
  
Posts: 188
Registered: 7-7-2012
Location: East TN / Near Oak Ridge
Member Is Offline
Mood: Awaiting Results....
|
|
Tis pretty, but I would hope to NEVER see than needle move in my presence 
<!-- bfesser_edit_tag -->[<a href="u2u.php?action=send&username=bfesser">bfesser</a>: removed
quoted images]
[Edited on 24.1.14 by bfesser]
A question that sometimes drives me hazy: am I or are the others crazy?
Albert Einstein
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Distilling bromine, always beautiful  This was to produce ~100ml. This was to produce ~100ml.
[img]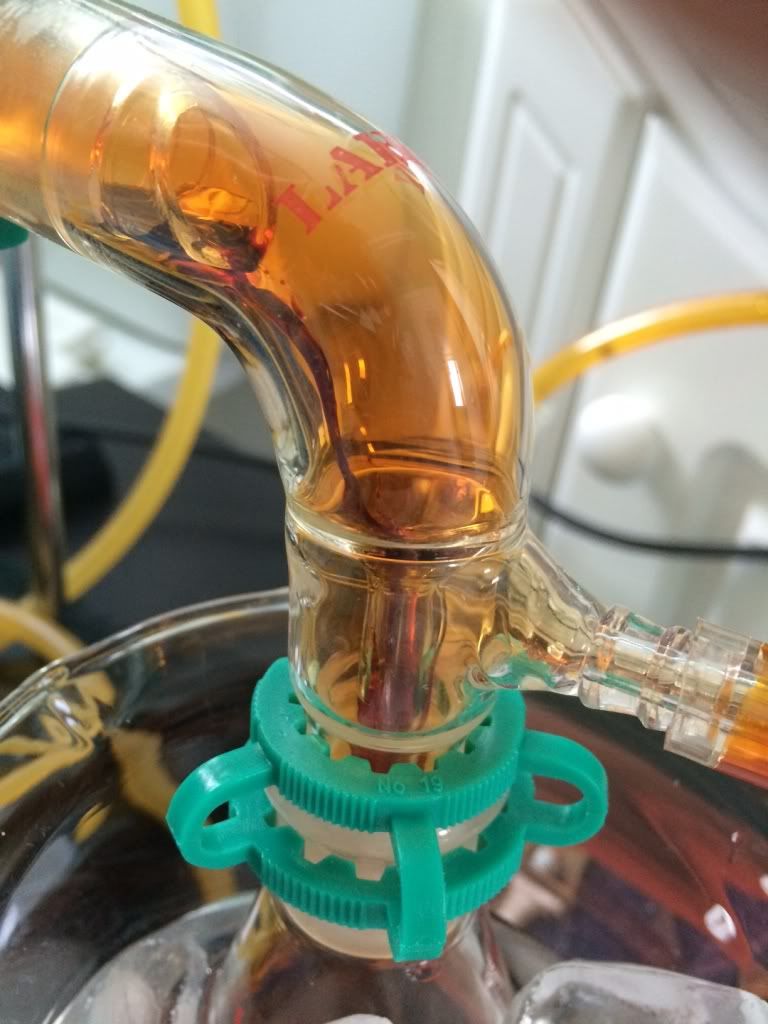 [/img] [/img]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Some astronomy: the moon, Saturn and Jupiter
  
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
Failed attempt to make a prince rupert's drop, beautiful anyway.

|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
How did it fail? The air bubbles inside? Looks awesome to me! I've always thought molten glass would shatter if dropped into RT water.
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
I don't know why it failed, all I know is that it did, when I crushed the tail the drop did not shatter. Second try : No bubble, a part of the bottom
of the drop shattered in the water. I'm done for today, it takes so long before the drop starts falling.
[Edited on 30-1-2014 by alexleyenda]
|
|
|
zenosx
Hazard to Others
  
Posts: 188
Registered: 7-7-2012
Location: East TN / Near Oak Ridge
Member Is Offline
Mood: Awaiting Results....
|
|
New pics
First are a CD pen type exposure counter after being reset and stored with my Uranium collection for about a year. There is also 1 uCi of Americium
there, but being a Y only emitter, it doesn't count. Total U ore = about 1 lbs.
While it took over a year, I certainly wouldn't want to sleep with that under my pillow. 20 R exposure would certainly cause quite a bit of DNA damage

Last photo is an extract of ~ 70 mL artificial vanilla which listed ethyl vanillin as the vanilla ingredient. Extracted with DCM.
The two spots look suspiciously like bacterial contamination.
  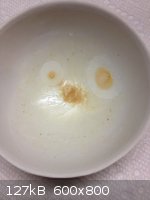
A question that sometimes drives me hazy: am I or are the others crazy?
Albert Einstein
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Beautiful flame of copper chloride/methanol. Interestingly, copper chloride is reduced in the flame to the metal. The second photo is of the
sulfate/methanol mix. It doesn't dissolve well (sulfates don't dissolve in alcohols). The chloride flame is ethereal and has a bluish tint that the
sulfate flame does not have.
The third photo is terbium sulfate filtrate under shortwave UV light.
  
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
ParadoxChem126
Hazard to Others
  
Posts: 104
Registered: 5-4-2013
Location: USA
Member Is Offline
Mood: No Mood
|
|
Steam-distilling crude chlorobenzene out of Sandmeyer reaction waste.

|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Hey, that's a good idea, to put weights on your chemistry stand, I'm going to do that next time I'm holding something heavy on it.
|
|
|
zenosx
Hazard to Others
  
Posts: 188
Registered: 7-7-2012
Location: East TN / Near Oak Ridge
Member Is Offline
Mood: Awaiting Results....
|
|
I was thinking the same thing when I saw that....
A question that sometimes drives me hazy: am I or are the others crazy?
Albert Einstein
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Precipitating copper carbonate, looks way better in real life.

|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
How could you live without weights on your stands :p The first thing I did when I received mine is to put weights on them, they are so unstable
without them.
@ Zyklonb, it looks great, I always loved copper solutions/compounds, the colours are so vibrant.
Anyways, here's my collection of canadian dollars vandalized through electroplating/heating/chemical plating  . It was a lot of fun/learning/challenge/frustration through many months, it is not really hard but I started doing
this 5 months ago when I just started chemistry as a hobby and I also had to wait to get more chemicals and labware to make different platings. The
next one will be silver when I'll finaly manage to get HNO3 to dissolve it, Nitrates are so hard to get in Canada! . It was a lot of fun/learning/challenge/frustration through many months, it is not really hard but I started doing
this 5 months ago when I just started chemistry as a hobby and I also had to wait to get more chemicals and labware to make different platings. The
next one will be silver when I'll finaly manage to get HNO3 to dissolve it, Nitrates are so hard to get in Canada!
The one on the left (normal) should be bronze according to wikipedia. The mg's color is strange, it's the first one I made back at the time, I guess I
did something wrong !
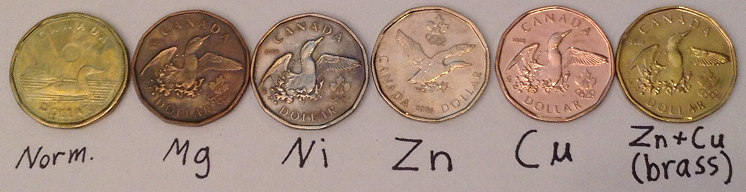
[Edited on 4-2-2014 by alexleyenda]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
What was the process for plating Mg? If you did it in water, the metal would not reduce - it would convert to the hydroxide instead because the water
already releases enough protons to reduce the metal. You'd need an anhydrous solvent like THF or pyridine.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
| Pages:
1
..
4
5
6
7
8
..
77 |