| Pages:
1
..
4
5
6
7
8
..
11 |
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
First of all allow me to apologize for the size of these photos,it exceeds forum limits so I was forced to remove the JPG ending, just download them
and rename it with the jpg ending. , if someone can at the very lest show me how to resize them inline I will edit this but I am forced to present
these pictures because like any good scientist It is my duty to back up claims made by myself and where as I applaud Blogfast for his valiant effort I
knew there where minor and crucial alterations being made effecting the out come of the reaction.
Here are two photos of freshly prepared solutions from the coins made by dissolving them in H2O2 and HCl.
I slowly dripped in Ammonium hydroxide solution so that the reaction can be observed as two layers where the boundary was where the reaction was
taking place. You can clearly see two very useful bits of information in them. You can clearly see the green precipitate falling out of the solution
and up top the copper amine complex.
This my friend is case closed , My mind can now rest relatively peacefully
tonight. Now on to the next challenge of using this as nothing more then a final wash to remove trace Cu left over from the electro-deposition. , My mind can now rest relatively peacefully
tonight. Now on to the next challenge of using this as nothing more then a final wash to remove trace Cu left over from the electro-deposition.
Attachment: GEDC1850 (1.7MB)
This file has been downloaded 1167 times
Attachment: GEDC1844 (1.5MB)
This file has been downloaded 1032 times
Would right more but am in a hurry, enjoy 
[Edited on 19-11-2011 by Sedit]
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Sedit, here are your images posted inline - rotated and resized:


Very nice demo Sedit! Create an account with Picasa, Photobucket, etc. You can then post inline images using the appropriate tags.
[~img]link to your image goes here[~/img]
The '~' is so it will display properly as text. Remove it from each tag when posting pics!
Tank
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I would rather just try to download and image editor that is virus free. and stable on my piece of shit computer but until then I am forced to do what
I just did. Thank you for getting the pictures right. I just feel like a kewl talking out my ass the whole time I have been unable to present my work
in the form of a pictorial.
I will make this short because quite frankly, I indulged in spirits a little to much in the past few hours and typing is very very hard,
You can see the green precipitate that people are having trouble achieving clear as day so Blogfast declaration of game set match, checkmate has been
visually dis-proven and that was my main goal right now to ensure that it is known that my claim is valid.
Ok its bed time fellows good night, I hope you all gained some incite as to why we are all getting various results.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
cyanureeves
National Hazard
   
Posts: 744
Registered: 29-8-2010
Location: Mars
Member Is Offline
Mood: No Mood
|
|
is it morning yet? nice work gentlemen now if only the blue is indeed just copper and the green just nickel. maybe this only works with the hcl
method,either way its darn good.i quit the ammonia method because i electrolyzed in sulfuric acid solution and not hcl. but i think sodium hydroxide
would've dropped both the copper and nickel from my solution in form of hydroxides and further heating the precipitates with sodium hydroxide will
separate the copper from nickel when taken back to solution as the nickel will remain as insoluble hydroxide.nickel in its hydroxide should give me
nickel oxide if i roast it in open air. will a coin dissolved in either hcl and peroxide or sulfuric acid be the same as electrolyzing the coin in
the solutions?because using current is just much quicker.its awesome how nickel can make things so pretty and shiny and super hard.
[Edited on 19-11-2011 by cyanureeves]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
LOL, uggg, yeh its morning..... Funny how EtOH is a couple hours of fun then 24 hours of side effects  . Beer taste so much worse in my mouth the second day. . Beer taste so much worse in my mouth the second day.
My experiments in the past suggest that the green precipitate is nothing but the Nickle. Unless Copper chlorides can fit themselves into the crystal
structure of Nickle chloride then its highly improbable that there is copper contamination in the green precipitate.
You can see in the photos a transition from what appears to be the mixed hydroxides to the pure green nickle but then again that could be nothing more
then a trick of light as the green precipitate attempts to shine through the dark blue solution.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
@Sedit:
Perhaps the remainder of the spirits were still talking but please restrain from putting words into my mouth: I never wrote “game set match,
checkmate”, I wrote “game over”, not as a triumphant expression but as one of: ‘I’ve had enough of this’. Not once have I doubted your
word, but I was unable to replicate your results and doubted (and still do) any feasibility of actual, practical separation this way.
Using a slightly acidic (this is important!) solution of CuCl2/NiCl2, both approx. 0.2 M, I’ve managed to produce your green precipitate, even
though the following crap photo doesn’t do it justice:
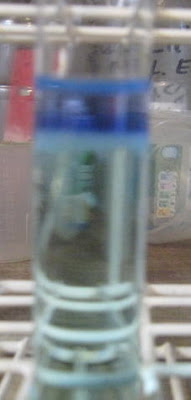
With the naked eye, the bottom of the interface between added ammonia solution and Cu/Ni solution can be seen to be a green precipitate. I added very
slowly the weak ammonia (0.5 to 1 M NH3) to the Cu/Ni solution and at some point, yes, this strange interface, including Ni(OH)2 precipitate and
copper ammine complex, occurs.
With hindsight, explaining what is going on here is not difficult and doesn’t contradict what I wrote. This is a highly non-homogenous,
non-equilibrium situation: at the interface the pH is just so that Ni(OH)2 precipitates, yet there is just enough free NH3 to complex the Cu.
On shaking everything dissolved and neither Ni(OH)2 nor the copper ammine complex survives because resulting solution is acidic: neither NH3 nor OH-
can survive in appreciable amounts.
To make this somehow the mechanism for effective separation between Cu2+ and Ni2+ is a pipedream, IMHO: it would require such accurate control of all
concentrations and pH, as well as heterogeneous conditions to make it work.
I don’t believe separation between various ammine complexes (Zn, Co, Cu, Ni…) on the basis of differential complexation and differences in
solubility of their hydroxides is actually viable, nor have I ever come across descriptions of such methods.
The model I put together applies (like most chemical models) in homogenous equilibrium conditions.
It's also no big surprise that we didn't obtain the same results at first: I was doing something very differently from you...
[Edited on 19-11-2011 by blogfast25]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I'm just busting your balls blogfast,  I do honestly appreciate your efforts
without a doubt. Many times people will discredit something and never once head to the lab to see for themselves and the fact you did quickly earned
my respect without a doubt. You seen a claim you did not feel was valid and at the speed of lightning setup to reproduce the experiment. That's not
just a good scientist that's someone with a love and a passion for what they do ::End Kissing ass:: I do honestly appreciate your efforts
without a doubt. Many times people will discredit something and never once head to the lab to see for themselves and the fact you did quickly earned
my respect without a doubt. You seen a claim you did not feel was valid and at the speed of lightning setup to reproduce the experiment. That's not
just a good scientist that's someone with a love and a passion for what they do ::End Kissing ass::
I noticed the same as well, once shaking happened the precipitate vanished. This is why it is so had to duplicate these results. The scale I was
running it in in the past was much higher leaving alot more room for error when it comes to concentration. If the concentration of NH3 to chloride
solution is just right the Nickle hydroxide will precipitate out. Any more and its complexed. Any less and it stays as the chloride salt.
I also question the practicality of my method but for different reasons. On a larger scale its very simple to achieve the stable precipitate
however... It is very hard to filter and given it should have multiple washes to ensure purity this is a huge hurdle. Because of this I feel it be
best you use it as a final wash of the electrochemical method and nothing more so that if your going to filter it lets just do it once and do it right
because it reminds me of trying to filter clay.... it just clogs up everything and hardly even settles making decantation a pain in the ass as well.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Ok, no hard feelings! 
It is quite remarkable, these ‘interface’ conditions, very peculiar…
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Yes the interface is just clear evidence of how concentration is highly effecting the outcome of the reaction.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by cyanureeves  | | will a coin dissolved in either hcl and peroxide or sulfuric acid be the same as electrolyzing the coin in the solutions? |
Not really. A well-controlled electrolysis will remove a significant amount of Cu. Further electrolysis with a carbon anode will remove the vast
majority of remaining Cu.
Simply dissolving the alloy in acid/peroxide will conserve the metal ratio so they must be separated by some other means. Note that Sedit and I
observed that some Copper Sulfate will crystallize from H2SO4/H2O2 so that's one soft exception.
Tank
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Yeh but dissolving them in H2SO4 takes so frigging long there is no way I would bother with H2SO4 and H2O2, its just a waste of time and reagents when
HCl and H2O2 can handle them over night for the most part.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
@Sedit, true but it can be sped up some by adding a bit of nitrate. Watch the fumes (from a distance). 
For those interested in precipitating nickel with aluminum:
A word of caution about the grade of aluminum used. Most aluminum foils will give mixed or bad results. I set up a simple experiment to demonstrate
this. I used approx 10mL of electro-refined Nickel(II) Chloride in each shot glass. Food grade Al foil was added to one while pure Al drill shavings
were added to the other. The precipitates were rinsed equally well and left in water to photograph.
Foil-precipitated nickel on left. Pure Al-precipitated nickel on right:


Weird/beautiful magnetic nickel flower (still under water).

I'm glad I didn't precipitate the whole lot of electrolyte with Al foil. Both precipitates were about equal in their strong attraction to a magnet. I
can't help wondering what component of the alloyed Al gives the reddish color.
I'll be conducting a few experiments with ascorbic acid. I would love to finally be able to say with some certainty how much copper remains in the
electrolyte after refining. If anyone can save me a little time on this, I'd love to read your suggestions.
Thanks,
Tank
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by m1tanker78  | I'm glad I didn't precipitate the whole lot of electrolyte with Al foil. Both precipitates were about equal in their strong attraction to a magnet. I
can't help wondering what component of the alloyed Al gives the reddish color.
Tank |
Colloidal copper, most likely.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Oh my god do I have great news for all of you.
Check this picture out and please resize and inline if feeling incline to do so.
Check it out. I diluted the Copper/Nickle dissolved in HCl a bit, at this time it was a brown solution that looked green when in trace amounts like a
drop.
So I carefully added Ammonium hydroxide and low and behold I achieved this what you see in the picture. Prior to the Dark blue copper amine complex
forming all of the Nickle precipitates out as the hydroxide. Notice the green solution on top and the large quantity of green precipitate on the
bottom.
Tell me, do you seen anything that suggest that this is the mixed hydroxides? The key seems to be to loss a small amount of nickle by keeping the
solution slightly acidic.
This is a MAJOR break through.
Attachment: GEDC1860 (1.7MB)
This file has been downloaded 1112 times
Attachment: GEDC1859 (1.8MB)
This file has been downloaded 1062 times
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Sedit, I don't think I follow. Did you use electro-refined nickel chloride or the mixed chlorides? If the latter, where's the copper? If you decant
the supernatant and rinse several times then allow the ppt to air dry, I'll bet you'll see results similar to mine (fluffy ppt dries to a tiny
pellet). The residue(s) left on the walls of the container as the ppt dries could provide some clues as well.
I had very similar results when I used strong ammonia. But then, the chloride solution was highly acidic IIRC.
Tank
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Tank, can you publish these photos above? I can't see them (no recognised file extension)
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
Sedit's images:


Tank
[Edited on 11-22-2011 by m1tanker78]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hmmm… that just doesn’t make any sense.
The Ks for Ni(OH)2 is 5.5 x 10<sup>-16</sup>
So, [Ni2+] x [OH-]<sup>2</sup> = 5.5 x 10<sup>-16</sup> … Eq.1
Also, at all times:
[H3O+] x [OH-] = 10<sup>-14</sup> = Kw … Eq.2
Isolate [H3O+] from Eq.2 and insert in Eq.1, then make explicit in (solve for) [H3O+], and take the negative logarithm:
pH = - log [H3O+] = 6.37 - ½ log [Ni2+] ( = - ½ log (K<sub>w</sub><sup>2</sup>/K<sub>s</sub> - ½ log [Ni2+]) - ½ log [Ni2+])
This is the minimum pH needed to keep a given concentration of Ni2+ in solution without any precipitation to occur:
[Ni2+] = 1 M … pH < 6.37
[Ni2+] = 0.1 M … pH < 6.87
[Ni2+] = 0.01 M … pH < 7.37
[Ni2+] = 0.001 M … pH < 7.87
For Cu(OH)2, Ks = 4.8 x 10<sup>-20</sup>, repeat the above and get:
pH = - log [H3O+] = 4.34 - ½ log [Cu2+] ( = - ½ log (K<sub>w</sub><sup>2</sup>/K<sub>s</sub>
No surprise there: Cu would start precipitating first, from lower pH values because Cu(OH)2 is much less soluble. This is quite similar to the
separation of Cu and Ni based on difference in solubility between their sulphides.
Complexation in these conditions can be neglected because [NH3] ≈ 0
Sedit, there’s gotta be something wrong there.
Edit:
Oookaaayy: long calculation but in these photos there's no copper whatsoever, if you ask me! 
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
My thought exactly. If he pre-refined the chloride then I guess it makes sense. If not, he has some copper hunting to do!
EDIT:
http://en.wikipedia.org/wiki/Dicopper_chloride_trihydroxide
| Quote: | Cu2(OH)3Cl can be prepared by hydrolysis of a CuCl2 solution at pH 4 ~7. A variety of bases such as sodium carbonate, ammonium, calcium, or
sodium hydroxide may be used (eq. 3).[1]
2CuCl2 + 3 NaOH → Cu2(OH)3Cl + 3 NaCl (eq.3)
Cu2(OH)3Cl can also be prepared by the reaction of a hot CuCl2 solution with freshly precipitated CuO (eq. 4).
CuCl2 + 3 CuO + 3 H2O → 2 Cu2(OH)3Cl (eq.4)
If sufficient chloride ions are present in solution, hydrolysis of CuSO4 with alkali also produces Cu2(OH)3Cl (eq. 5).
2 CuSO4 + 3 NaOH + NaCl → 2 Cu2(OH)3Cl + 2 Na2SO4 (eq.5)
|
It's insoluble to boot. I wonder if this compound has been overlooked?
Tank
[Edited on 11-22-2011 by m1tanker78]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Tank:
The one thing that could 'mask' the copper is a lot of chloride: chlorocuprate anions (CuCl<sub>4</sub><sup>2-</sup> then form and these are emerald green. But it needs quite a lot of
Cl<sup>-</sup>. My solutions (above) are in fact mixed CuCl2/NiCl2 and they're blue. The complexation constant of the chlorocuprate
complex is fairly small. then form and these are emerald green. But it needs quite a lot of
Cl<sup>-</sup>. My solutions (above) are in fact mixed CuCl2/NiCl2 and they're blue. The complexation constant of the chlorocuprate
complex is fairly small.
No, Cu2(OH)3Cl hasn't really been overlooked: it's just that in borderline conditions, Cu2+ + 2 OH- === > Cu(OH)2 is a bit of an
oversimplification: the hydrolysis of Cu2+ happens in stages. At higher pH the Cu2(OH)3Cl reverts to Cu(OH)2 'proper'.
Imagine it like this:
Cu(H2O)<sub>n</sub><sup>2+</sup> + H2O === > Cu(OH)(H2O)<sub>n-1</sub><sup>+</sup> +
H3O<sup>+</sup>
And again. With Cl- these subspecies can then form hydroxy chlorides, like the one you mentioned and these are often insoluble. Fe3+ does it too, more
so even due to the higher charge and stronger associated central electrical field, which leads to de-protonation of the water cloud...
I'm afraid this is not a day of 'break throughs' 
There remains one possibility: if he's saturated a Cu2+/Ni2+ solution with chloride, then most copper will be present as green chlorocuprate complex
anions, indistiguishable from Ni2+. That would offer slight protection against hydrolysis. But add NaOH to a solution containing chlorocuprate and
Cu(OH)2 drops out all the same: the complex isn't strong enough and Cu(OH)2 (or copper (II) hydroxy chlorides) too insoluble. Perhaps with weak NH3?
Doesn't sound convincing to me.
I'm gonna check this out... Is his green precipitate in this case a copper hydroxy chloride?
[Edited on 22-11-2011 by blogfast25]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I really don't know what to make of it yet honestly. It shocked me to see so much precipitate without the appearance of the amine complex.
The filtrate did have a slight blue tinge to it but that could have been a play on light I'm not sure.
I washed the precipitate with cold clean water a couple times then just added HCl to gain a dark green solution I'm boiling down. There is a slight
red tinge to the reduced product after the addition of Aluminum so there is some chance that Copper made its way in. This could just be an artifact of
poor washing however or it could be something more only further experimentation will tell.
I'm going to go do some more work and see where it gets me, be back in a few hours with hopefully more pictures to show.
[edit]
| Quote: | 
My thought exactly. If he pre-refined the chloride then I guess it makes sense. If not, he has some copper hunting to do! |
The solution I filtered off of the precipitate I slowly added more Ammonia to expecting to recover a small amount of extra hydroxide... instead it was
just a rapid copper amine complex...
Don't stress it, im as confused as yall are at the moment. This was not what I was expecting at the start of the experiment.
[Edited on 22-11-2011 by Sedit]
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I dispensed about 20 ml of 36 % HCl in a beaker and started dissolving the 1:1 Cu:Ni mixed hydroxide into it. At first the solution was pretty green,
due to the excess chloride, then it slowly became more blue. And at some point during the additions a blue-green, sandy (not gelatinous) precipitate
formed. Stirring didn’t dissolve it. This is almost certainly Cu2(OH)3Cl (or Cu(OH)2.Cu(OH)Cl, rewritten) It looks very much like ‘Verdigris’,
basic copper carbonate (CuCO3.Cu(OH)2). Adding a little more HCl dissolved it (hence no photo).
Side note: if the pH at which this hydroxy chloride forms is low enough, separation between Cu2+ and Ni2+ should be possible that
way. Will check that tomorrow.
Some more mixed hydroxide was the added until the blueish solution below was obtained:

In that I dissolved a good dollop of NaCl and the green of chlorocuprate anions reappeared:

To this I added weak ammonia in a test tube:

This is the same phenomenon as before: interface lack of homogeneity/equilibrium. That precipitate is gelatinous and greener than the earlier observed
precipitate.
But it should be possible to precipitate Cu2(OH)3Cl from this solution with careful addition of NaOH…
[Edited on 22-11-2011 by blogfast25]
|
|
|
m1tanker78
National Hazard
   
Posts: 685
Registered: 5-1-2011
Member Is Offline
Mood: No Mood
|
|
He mentioned this...
| Quote: | | The key seems to be to loss a small amount of nickle by keeping the solution slightly acidic. |
...which is what aroused my suspicion that this could be the hydroxy chloride of copper (among other things). I skimmed through your equations and
your experimental findings (will read more judiciously later). Your last experiment seems to reinforce the fact that the ppt only exists in a narrow
[slightly acidic] pH range.
Maybe this helps to explain one of my previous observations that I dug up from pg. 3 of this thread. I plated nickel from what was supposed to be the
copper-ammonia complex. I later discovered that by changing the current density, an inferior copper deposit could be obtained as well. (edit) In
retrospect, it could have been that Ni2+ was depleted - not so much to do with changing the current density.
Quote: Originally posted by m1tanker78  |
Contrary to what has been proposed and reported before, the experiment clearly demonstrates that nickel ions are present, possibly in abundance, in
the ammonia complex. The proportion of copper ions (if any) of the same is unclear as of yet.
[...]

|
Tank
[Edited on 11-23-2011 by m1tanker78]
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Its copper precipitate of some sorts even though at first sight it had me convinced it was no doubt some form of Nickle precipitate. I established
this by turning the precipitate into its chloride and then adding Aluminum.
As the hydrogen rapidly gassed off I held a flame over it and it gave burst of blue flames where as to the best of my knowledge Nickle should give no
color in the flame test.
Sorry for getting people hopes up but you can see from the photos where my excitement came from,that precipitate looks exactly like Nickle hydroxide
and even though the reduced product after my little flame experiment proved there was some Nickle in there it also showed heavy Copper contamination.
I have the chloride solution drying as we speak so when its dry and crystallized it will make it much easier to determine the true contents of the
large amount of green precipitate.
I vow that this is not over yet and I will conquer this one but curve balls and the unexpected seem to occur around every corner
PS: Blogfast when I post my pictures and it ask what program to use to open them just use Internet explorer it will open them with ease. Either that
or just add a JPG extension to the file.
[Edited on 23-11-2011 by Sedit]
What a sick sick world we live in with this hobby.
The wise chemist thought to himself " I know, I will just sprinkle in some NaOH and the precipitated hydroxide from that should tell me once and for
all if its copper of some kind or Nickle of some kind...."
So the chemist did just that expecting to see either blue or green precipitate.... Instead the chemist found himself with an opaque brown
solution. As if this whole set of nonsense couldn't get much stranger to the
chemist this happens. As if this whole set of nonsense couldn't get much stranger to the
chemist this happens.
Pictures in the morning its late now. ::Sigh::
[Edited on 23-11-2011 by Sedit]
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Sedit, thanks for the tip on reading your pic files.
I added 1 M NaOH dropwise to the green solution (CuCl2/NiCl2/NaCl, pH about 3) above, from a burette. The first precipitate kept redissolving on
stirring but from about pH = 4 (paper) permanent precipitation occurred. It took quite a bit of patience to carefully get to pH = 5 but precipitate
kept on forming. Due to a mishap I then added slightly too much and stopped: It looked like this by then:

This was filtered and the filtrate was clear and colourless. The pH meter gave pH = 7.9 and not surprisingly no further precipitation occurred on
adding more NaOH: the nickel had precipitated too, showing that as a separation method, if at all possible, this would require very accurate pH
control, probably buffering. (And beware of possibly irreversible co-precipitation of Ni(OH)2!)
This precipitate was profusely washed with hot DIW and the last wash water then tested for chloride with lead acetate solution (silver nitrate would
be better but I haven’t got any). No precipitation was observed (see left tube, bottom pic). This is the filter cake after washing, slurried in a
bit of DIW:

1 M NaOH was then added to a slurry of the filtercake, it turned blue and more fluffy, presumably because of conversion of the copper hydroxychloride
to copper hydroxide, liberating the chloride as NaCl (pH was now >> 7). It was heated slightly:

This was filtered and the first filtrate intercepted, then acidified with acetic acid to pH ≈ 4 (paper) and strong PbAc<sub>2</sub>
added. Turbidity developed immediately, see right hand tube:

To be honest I had expected a stronger precipitation of lead chloride, as this would be stronger evidence that the original filter cake contained
chemically bound chloride. But pure Cu2(OH)3Cl only contains about 17 % Cl and the precipitate also contains Ni(OH)2 of course and there’s lot of
water too. I may repeat this experiment in more controlled conditions, and without Ni present, with dried Cu2(OH)3Cl.
All the green precipitates we've seen in the above posts are likely to be copper hydroxychloride. Tank was right: we really did overlook the
role chloride seems to play here...
[Edited on 23-11-2011 by blogfast25]
|
|
|
| Pages:
1
..
4
5
6
7
8
..
11 |