| Pages:
1
..
4
5
6
7
8
..
10 |
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Well..... *ouch my head* hmmm... It does have a lid, a very nice one with a small hole in its center.
Don't do like me, propane burning and drinking just don't mix well...
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
IKEA
Btw, yes, the outer shell for the propane burner really IS an IKEA trashbin. I suspect they never anticipated this use of it......
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
sulfur burner using an aspirator
Axehandle I hope your hangover is over. Even Norse gods must take a rest. Here are some more musings on your most interesting project:
I have another thought for a sulfur burner. Since I've read that one industrial method for burning sulfur to SO2 is to atomize molten sulfur and
mix it with air, why not use an aspirator with air as the motive fluid to do this. These aspirators are available for industrial use and are known as
"jets". They are made by Penberthy-Houdaille and others. I have used one as a pump using steam as motive fluid at work. Even the smallest
of these (1/2" would be too big for your burner but you could get some design
parameters from them and then make your own as you have your own industrial laboratory. This might be an effective sulfur fired torch. would be too big for your burner but you could get some design
parameters from them and then make your own as you have your own industrial laboratory. This might be an effective sulfur fired torch.
My references also note that the exiting sulfur dioxide/oxygen ratio is important for efficient SO2 production. I believe that is why I was required
to determine the oxygen content of the sulfur burner effluent using an Orsat analyzer at the sulfite paper mill I worked at a long time ago.
|
|
|
BromicAcid
International Hazard
    
Posts: 3272
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Maybe add some KNO3 to the mix or other oxidizers in small amounts, just to modify the burning properties of the mixture, expecially the candle.
However I might have said this already as I've been thinking about this for awhile, and this thread is getting unmanageably large.
|
|
|
Al Koholic
Hazard to Self
 
Posts: 98
Registered: 2-12-2002
Member Is Offline
Mood: Seeking ligand
|
|
Yeah, it is getting quite large...but very enjoyable.
I gotta ask...axehandel...you listen to any black metal? I mean, if you weren't, with your hobby, and your locale, I'd be surprised.
Anyway, keep up the good work!!! Very good thread to be following!!!!!!!!
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Actually..... yes. I do listen to black metal. How did you guess?
On a more interesting note, I built a simple ozone generator today using a drinking glass, some aluminum foil and a 9kV NST. The corona discharge was
a very beautiful dazzling blue. I didn't dare to keep it on for long though, not until I've checked up on the harmfulness of ozone more
scientifically (i.e. not by sniffing it until I drop to the floor).
Tomorrow I get my heating coil for the exhaust heater. I also intend to cast the aluminum burner vessel tomorrow. I've had very promising results
with the rockwool substrate. Expect a bombardment of pictures. Hmm, local time is 03:46. Perhaps it's time to get some sleep? Expecially since
the air in here suddenly became very fresh. Hey, if I build this huge ozone generator I could skip showers alltogether! Ozone removes odors! Great
idea! No need to wash my clothes ever again, either.
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
ozone vs health
I offer some anecdotal observations on ozone vs health. Many years ago my grandmother and her 2nd husband had an ozone generator in their living
room. When I came over to visit them the place always reeked of ozone. They had rheumatism/arthritis or such and the ozone purportedly helped
relieve it.
I did not inspect the generator too closely at the time but I remember that it was composed of 5 - 10 parallel glass tubes which took on an eerie
purple/pink glow when electrically powered. My impression was that this never really helped them any but it didn't seem to hurt them any either.
|
|
|
Hermes_Trismegistus
National Hazard
   
Posts: 602
Registered: 27-11-2003
Location: Greece, Ancient
Member Is Offline
Mood: conformation:ga
|
|
DON'T GIVE UP ON COMBUSTION!!!
Burning a fine spray may not be as difficult as it sounds at first.
Have you ever seen those pneumatic cannon's people build out of PVC or steel pipe?
if not look at Slava's site... http://www.voltsamps.com
http://www.gizmology.net/airgun.htm
http://members.aol.com/sph911/spud/basic.html
anyway, they basically consist of a barrell, an air resevoir, a nipple to hook up a small air compressor (often a small 12V car compressor) and a ball
valve to connect the resevoir to the barrel.
anyway, if you made the air resevoir large, fixed a filling port to the barrel to admit sulfur, wrapped a heating element around the barrell to bring
the sulfur up to liquid temperature and made a tiny hole on the end cap covering the barell opening.......
you'd be in business my friend!
Arguing on the internet is like running in the special olympics; even if you win: you\'re still retarded.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Thanks for the input. I don't really intend to use the ozone generator for anything, really.... I built it just due to the amusement factor.
And aaah, yes, spud guns. I built something similar once, only it didn't have a plastic, but a steel barrel, and it didn't use a fuel-air
mixture as propellant but black powder, and it didn't shoot a potato but a lead ball, but the principle holds.. 
Using boiling sulfur to feed a nipple with a small hole in it much in the same manner as my propane burner works, perhaps without a fan even, might be
worth some serious investigation. The brass nipple would have to be replaced with an aluminum one ofcourse.... and I shall need an aluminum rod,
but... hmm. This is the perfect time to speculate, I'll probably make the exhaust heater today and the burner tomorrow.
Edit: I'm seriously considering using the boiling sulfur to pump gaseous sulfur through the burner nipple. I think I'm missing a caveat here
though. Better think it through _really_ carefully.
Edit2: Ah. The caveat is the possibility of the boiler blowing up. Better put a burst diaphragm in there somewhere. It will also be a bit tricky to
ignite the fuel-air mixture in a closed system.
Edit3: Off I go to get my heating coil!
[Edited on 2004-3-29 by axehandle]
[Edited on 2004-3-29 by axehandle]
[Edited on 2004-3-29 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
What the fuck is wrong with my sulfur???
ARRRRGGHH!!!!
I tried (again) to heat the sulfur to its auto-ignition point (250C-ish). The digital thermometer I used topped out at 300C (its maximum). I used a
glass tube connected to an air pump to bubble air through the molten sulfur, to aid the ignition process.
RESULT: <b>No fucking fire whatsoever!</b> Something is very WRONG here.
Anyone with lots of sulfurious experience?
I'm depressed now. I'm going to play SOF2 instead.
[Edited on 2004-3-29 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Hermes_Trismegistus
National Hazard
   
Posts: 602
Registered: 27-11-2003
Location: Greece, Ancient
Member Is Offline
Mood: conformation:ga
|
|
If the glove doesn't fit.......you must aquit.
and if empirical evidence doesn't fit the published data, then the data must be faulty.
P.S. section 9 of this MSDS, lists the auto ignition temperature of Sulfur to be 450 Deg Celcius.
http://avogadro.chem.iastate.edu/MSDS/sulfur.htm
Arguing on the internet is like running in the special olympics; even if you win: you\'re still retarded.
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
I used to (and sometimes still do) make sulphurous acid by heating sulphur on a spirit burner (usinf EtOH). The sulphur was placed in a container. A
glass pipe was placed nearly touching the lower end of the container and another outlet pipe was attached to the upper end. The sulphur was heated
till melted and air was pumped through the liquid using an aquarium air-pump (the ons they use to bubble air). I normally didn't see any fire in
there, by the gas comming out surely smelled of SO2. The gas was then dissolved and tests were made on salts prepared from the acid. It was sulphurous
acid. Now I cannot understand how your sulphur would not combust. Did you try bubbling some of the gas which escaped through water and testing with
litmus or any pH indicator?
[Edited on 29-3-2004 by Esplosivo]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Bzzzzt: Wrong! It's 450 deg Fahrenheit, which equals 232.22 deg C. Check again.
And hmmm no, I didn't bubble it through water. My nose clearly tells me there was little or no SO2 there though...
Well, when all other explanations are shown to be faulty, the one remaining must be correct.
I submit that there is something wrong with my sulfur. I'll try to repeat the experiment with sublimed (not mined) sulfur instead. I think I have
some left.
[Edited on 2004-3-29 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Hermes_Trismegistus
National Hazard
   
Posts: 602
Registered: 27-11-2003
Location: Greece, Ancient
Member Is Offline
Mood: conformation:ga
|
|
OH.....
Ok...I guess I just sorta skimmed it through....
Arguing on the internet is like running in the special olympics; even if you win: you\'re still retarded.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Don't worry, it happens to me all the time. It's always embarrassing though... 
Edit: Well, I'm casting the plaster mold for the burner now. It will be shaped like a hollow cylinder, whatever the correct geometrical term for
that is.....
Edit2: OK, this is REALLY weird. The sublimed sulfur didn't ignite either! Until I was about to pour it into a large bowl of water, that is. THEN
it caught fire, right in the pot! I'm stupified.
[Edited on 2004-3-29 by axehandle]
[Edited on 2004-3-29 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
Auto ignition point is a theoretical and empirical value which corresponds the lowest temperature where ignition has been observed , in air usually
stated.
It doesnt necessarily means that it will be easy to ignite. Strangely though, since sulfur has a flash point of roughly 150 C. But for a risk analysis
, say for a molten sulfur 10000 MT bulk ship this is important...dont want any spillage of molten sulfur hitting an uninsulated exhaust pipe and
catching fire or something similar...
I remember reading that the SO2 content in the air above the sulfur increases autoignition point dramatically, which could explaing for the easier
ignition in a open area.
I have never had any problems with igniting sulfur in the open in small metal cans, using matches (use the SO2 for sterlilizing) , it is surprizingly
easy to ignite with a match according to me, and burns with a very nice blue flame .
/rickard
[Edited on 29-3-2004 by rikkitikkitavi]
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Because, it eliminates one step from the process. I admit that I could just as easily ignite the molten sulfur by hand, but it wouldn't be as
aesthetically pleasing.... 
I have one more card up my sleeve. A ceramic igniter (a heating coil embedded in clay, capable of making the clay rod glow). This is problematic for
one reason: Where to draw the connection wires? I think I'll simply resign and do it the oldfashioned way, i.e. using a match...
My biggest problem is that I'm a perfectionist.
Edit: I'll go and finish my mold now...
Edit2: Note to self: Don't make a plaster mold in cookware with plastic handles. Drying it out in the oven without melting the plastic takes
forever. I am an idiot.
Edit3: Very soon to reinstall win2k on this machine. Then it's ALUMINUM CASTING TIME!!!! YEAH!!!
Edit4: | Quote: |
I remember reading that the SO2 content in the air above the sulfur increases autoignition point dramatically, which could explaing for the easier
ignition in a open area. |
It would certainly explain why the sulfur caught fire as soon as I started pouring from the melting pot into the vat of water. Very unreliable stuff,
this. Thanks for the info.
[Edited on 2004-3-29 by axehandle]
[Edited on 2004-3-30 by axehandle]
[Edited on 2004-3-30 by axehandle]
[Edited on 2004-3-30 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
BromicAcid
International Hazard
    
Posts: 3272
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Axehandle, remember the digital library of India? They've got a book called Raw Materials for The Manufacture Of Sulphuric Acid And The Manufacture Of Sulphur Dioxide and around page 316 it starts to detail sulfur burner
designs and such. Also it has some other interesting information on sulfur, more then necessary but very interesting.
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Sulfur burners.
Yes, I've studied what I could find on the net, but I forgot about the digital library. Most of the burners I've found don't lend
themselves to downscaling easily.
But tonight, I have cast the burner bottom! I didn't use gloves, but managed to only get one 1st degree burn. (And I set fire to the table when I
spilled some glowingly hot Al on it  --- easy enough to put out though...) --- easy enough to put out though...)
No pics of the burner bottom yet (it has to cool first) yet, but here's the result of pouring the Al leftovers into water:

My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Saerynide
National Hazard
   
Posts: 954
Registered: 17-11-2003
Location: The Void
Member Is Offline
Mood: Ionic
|
|
Very artistic 
|
|
|
axehandle
Free Radical
    
Posts: 1065
Registered: 30-12-2003
Location: Sweden
Member Is Offline
Mood: horny
|
|
Bottom done!
Here is the cast bottom, made of pure (99.9%) Al. It would look shinier if I polished it 
The twisted thing inside is for distributing the heat better when the burner is in use.

Oh, and here is the inside:

Edit: I'll be going on vacation now for about a week. I may not have internet access.
[Edited on 2004-3-31 by axehandle]
[Edited on 2004-4-1 by axehandle]
My PGP key, Fingerprint 5D96 E09E 365D 1867 2DF5 C2FE 4269 9C19 E079 CD35
\"Verbing nouns weirds the language!\"
|
|
|
Tacho
National Hazard
   
Posts: 582
Registered: 5-12-2003
Member Is Offline
Mood: No Mood
|
|
This stuff is humiliating...
When I think of myself melting a few drops of Al in a tuna can, with pliers and a blowtorch... well, I feel pathetic. There must be 2kg of Al in that
thing!
Now, let me understand: You are going to half fill this thing with sulfur, set fire to it somehow, close with a lid and pump air through it. The
air+SO2 goes into a heater and then into the V2O5 tube than into water. Is that right?
BTW, I predict a huge suckback (is this expression right?) if the final tube goes INTO water. Would be better NEAR a water surface. I guess you
figured that.
I don’t want to spoil your foundry fun, but, to set things clear for future builders of your apparatus, why couldn’t you use a small aluminum
cooking pan? I have a few that already come with a lid that closes them almost hermetically
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
First of all its is interesting and I at least find it self-rewarding you make something yourself. Buying stuff doesn't make me feel so happy as
when I make it myself.
Secondly to avoid suck back he can use a set up as shown in the attachment. The use of an inverted funnel is stated when using gasses with a high
solubility in water or other solvent. I think the diagram is self-explanatory. Oo btw, I think axehandle had already figuered out the problem, but
just to give you an idea.
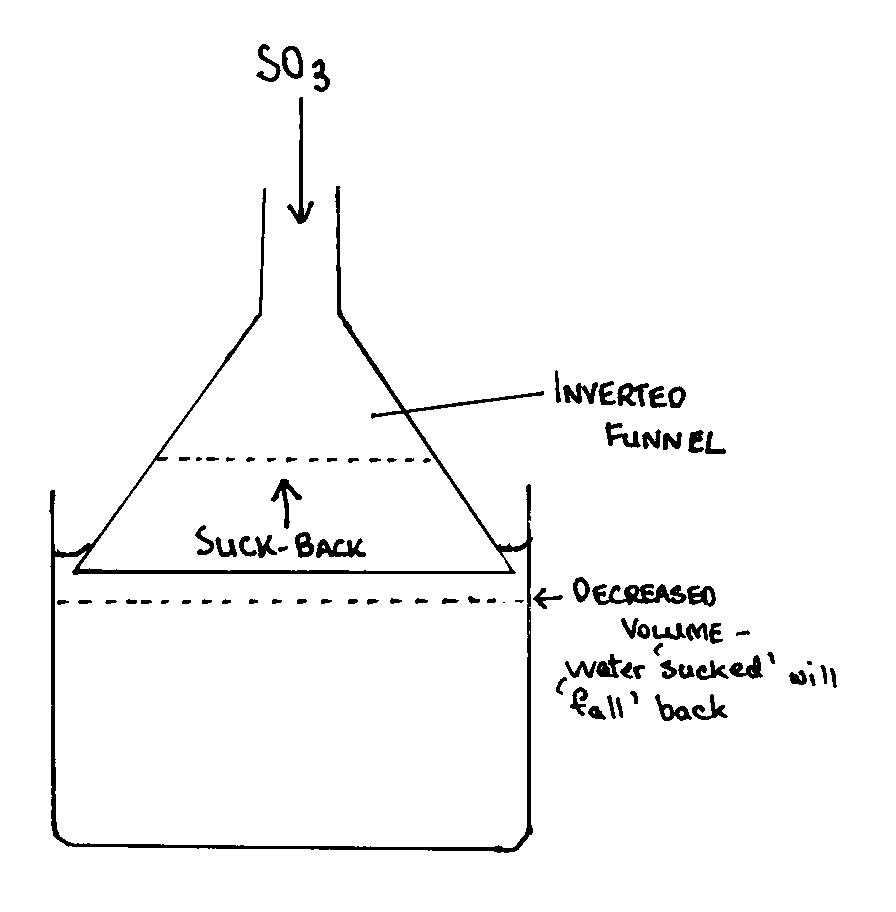
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
SO2 absorber
I have used the inverted funnel method in Organic Chem lab for HBr capture, IIRC. It worked well.
Of course the more industrial way to do this is with a countercurrent absorbtion column. But this would be more complex and require a pump capable of
handling H2SO4, say a peristaltic type (hose pump).
|
|
|
rikkitikkitavi
Hazard to Others
  
Posts: 192
Registered: 17-6-2002
Member Is Offline
Mood: No Mood
|
|
PVC is resistant to high concentrations of
H2SO4 when cold (20 C).
/rickard
|
|
|
| Pages:
1
..
4
5
6
7
8
..
10 |