| Pages:
1
..
4
5
6
7 |
-=HeX=-
Hazard to Others
  
Posts: 109
Registered: 18-4-2008
Location: Ireland
Member Is Offline
Mood: Precipitating
|
|
Anders: VERY interesting.
I had prepared 'some' Ethyl Perchlorate recently, and found a rather novel use for it.
Basically, I tested it as a filler in a micro-shaped charge, and it worked rather well. The cone was a .223 bullet jacket, and the charge was rather
tiny, tamped with corn starch/water. Initiation via EBW. 1cm standoff.
The charge neatly preforated the 1/8" target plate, proving to me that indeed, Ethyl Perchlorate is decent enough to form a jet. Too bad it is so
fucking sensitive.
If you give a man a match he will be warm for a moment. Set him alight and he will be warm for the rest of his life.
|
|
|
Anders Hoveland
Banned
Posts: 208
Registered: 15-6-2010
Member Is Offline
Mood: No Mood
|
|
The organic ester perchlorates are very sensitive because there is no extra electron resonating around in the oxygens (as in the perchlorate ion) to
stabilize it. The --NHClO3 group should theoretically be more stable, --NH(+)=ClO3(-), but it is still extremely sensitive. Perhaps having a
--N=ClO3(-) group and another --NH3(+) group on the same molecule would allow the incorporation of perchlorate without it being nearly so sensitive.
In my opinion a --C(NO2)3 group is pretty powerful (though not quite as much as --OClO3) and much more stable. Or consider
--NH2(+)OH ClO4(-) as a far more stable alternative if a perchlorate compound if wanted. Do not underestimate the nitro group. Remember, C(NO2)4 forms
very powerful expl. mixtures with other alkanes.
|
|
|
Blaster
Hazard to Self
 
Posts: 54
Registered: 7-11-2003
Location: UK
Member Is Offline
Mood: perchloric!
|
|
Hi to everyone. After a long time away I'm back.
I'm not missing any fingers or anything, ha ha!
Great to see this thread is still alive 7 years on.
I drifted away from Chemistry and additionally I changed ISP yet again to one which did not provide webspace, so my Perchloric Ester website
disappeared for a year or so.
After some U2U requests I've just re-instated it with some difficulty. It was written a long time ago on MS Frontpage but I've retained its original
layout.
I don't seem to be able to edit my earlier posts to change the link, but here is the new one:
http://www.cdodgyd.heliohost.org/perchloric/perchloric.htm
[Edited on 13-7-2010 by Blaster]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
Thanks for uploading those links again.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Here's a download link for the pdf of the 1960 ACS monograph on perchlorates by Shumacher
http://www.archive.org/download/pwechloratesthei001740mbp/pw...
Creatinine Perchlorate, (urinary perchlorate) is something I have been trying to find referenced anything concerning the energetic properties, but am
having no luck at all ....
piss on it 
[Edited on 3-8-2010 by Rosco Bodine]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Formatik  | No simple chlorate esters have ever been characterized or isolated. There are theoretical studies
(e.g. on methyl chlorate). Stettbacher estimated glycerin trichlorate would develop 3000cal/g
compared to 1580cal/g (heat of combustion) for the trinitrate ester. The material also isn't known. |
Given the properties described in the Ethyl Perchlorate references cited by Blaster
http://www.cdodgyd.heliohost.org/perchloric/hb3.jpg
" It explodes by ignition , friction , or percussion , and
sometimes without any assignable cause."
an even more sensitive chlorate would be impractical even as a lab excercise.
This is not to say that a usable explosive composition could not be made ,
by applying the means for synthesis without obtaining the product itself.
http://www.cdodgyd.heliohost.org/perchloric/ms5.jpg
" The ester was also obtained by adding 0.1 g of anhydrous
Silver Perchlorate to 4 cc of pure Ethylbromide."
" precipitating Potassium Perchlorate when reacted with KOH "
I previously noted explosive compositions based on double decomposition of reactants
http://www.sciencemadness.org/talk/viewthread.php?tid=5777#p...
A mixture of FH2CCH2F + 2 KClO3 => 2 KF + 2 CO2 + 2 H2O + Cl2
approximates an explosion of the hypothetical chlorate ester
Ethyleneglycol dichlorate (ClO3)CH2-CH2(ClO3)
heat of formation of 1,2-difluoroethane given here _
http://pubs.acs.org/doi/abs/10.1021/j100321a007
convert calories to joules , 4.186(- 102 ) = - 427 kJ / mol
. . 66 gm . . 2( 123.5 ) gm
FH2CCH2F + 2 KClO3 => 2 KF + 2 CO2 + 2 H2O + Cl2
. .- 427 . . . . . 2(- 398 ) . 2(- 567) + 2(- 394) + 2(- 242)
Subtracting enthalpies of reactants from products of explosion
- 2406 - (- 1223) = - 1183 kJ ∆He Heat of explosion
convert joules to calories , 1183 / 4.186 = 283 kcal
Dividing the ∆He Heat of explosion of the balanced equation by the molar weight
of the reactants (- 283 / 313 ) X 1000
obtains ∆He Heat of explosion = - 9 0 4 Kcal per kilogram of mixture
.

" estimated glycerin trichlorate would develop 3000cal/g "
must be highly endothermic to rate so high , Aluminum
and Dinitrogen Tetroxide will yield 3225 cal/g
.
[Edited on 4-8-2010 by franklyn]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
In the famous "spoon shot", does anyone know how Blaster initiated the drop of Ethyl Perchlorate?
|
|
|
Blaster
Hazard to Self
 
Posts: 54
Registered: 7-11-2003
Location: UK
Member Is Offline
Mood: perchloric!
|
|
I'll tell you myself - I held it above a flame briefly. The esters are extemely sensitive and I now consider myself lucky I suffered no unexpected
explosions (as the pioneers did back in the mists of time).
My overriding memory of the stuff is the sheer violence of its detonation. I was really taken aback despite having handled such compounds as NCl3 and
nitroglycerin previously. It just doesn't seem possible that such tiny quantities could cause such a blast; not to mention the noise - I had ringing
in the ears even with defenders on.
BTW, heliohost seem to be pretty unreliable. The site keeps going down. I may have to change again but I'll keep you informed naturally.
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Frankly I didn't know you still dropped by. I apologize for not making that a direct question.
Jeez....what a scary thing. I know you had all sorts of stuff on and it was only in the ml range, but... it's some scary stuff.
I can't remember if I have seen another "through & through" puncture from a sub-gram level materiel.....
Yea, I went to the site because it DID come up before. - I may just get some space for free that I was going to put up an FTP but if Madhatter still
has his I'm certainly not going to duplicate efforts. How much space are you looking for? I was thinking of putting up a page with about 1Gb of
firearms refinishing and simple repair; which would leave me a least 2-3 Gb (if I can get it for free - because I don't want to pay for it in an era
where a few Gb are generally free anyway). Supposedly a friend's son can get me 5Gb for free for 18months.
PM me if you haven't found a good deal.
[Edited on 5-8-2010 by quicksilver]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
| Quote: |
the perchlorate ester of hexene was prepared... by reacting iodine trichloride with acetic anhydride, with the subsequent addition of aqueous
perchloric acid and hexene.
|
Iodine trichloride actually exists as a dimer, I2Cl6, which is a dull orange solid melting at 63 °C. It is formed by simple reaction of solid iodine
and chlorine gas.
"Hexene" typically refers to 1-hexene, with the structure
CH2=CH-CH2-CH2-CH2-CH3
the "perchlorate ester of hexene" must be hexene-1,2-diperchlorate. No doubt ethylene gas could be used instead of hexene, but it is doubtful that the
resulting ethyl diperchlorate would be stable enough to isolate from solution.
| Quote: |
the reaction of ethylene oxide with [nitronium tetrafluoroborate] NO2BF4 and [lithium perchlorate] LiClO4 in ethyl acetate at 0-5°C gave
quantitatively 2-nitratoethyl perchlorate as a single product.
|
Kunglig Svenska vetenskapsakademien
The structure of 2-nitratoethyl perchlorate would be
O2N-O-CH2-CH2-O-ClO3
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Just a thought...has someone here some info about tert-butyl perchlorate?
Here are ideas I have for years now in mind...
I know that hypochlorous esters of primary and secondary alcohols undergo the following reaction in an explosive maner: yielding aldehyds and
ketons...
R-CH2-O-Cl --> R-CH=O + HCl
R-CH(-O-Cl)-R--> R2C=O + HCl
but ternary alcohol allow better stability of the hypochlorous ester...tertiobutyl hypochlorite is known and useful reactant in some organic
reactions...
So R3C-OCl is much stabler than R2CH-OCl and R-CH2-OCl!
The oxydability (lability) of the hydrogen atom on the carbon holding the O-Cl seems to be the problem.
From there I think that perchloric esters must have the same kind of stability problems...with as extra trouble the generation of concentrated HClO3
in an oxydable environment what is as everybody knows very bad news...
R-CH2-O-ClO3 --> R-CH=O + HClO3
R-CH(-O-ClO3)-R--> R2C=O + HClO3
Maybe tertiary alcohol would help entrap the power of perchloric esters in a safer way...
I have the idea that (CH3)3C-OClO3 must be a first step...trial
then pinacol diperchlorate O3ClO-C(CH3)2-C(CH3)2-OClO3 what must be denser and must display a better OB, thus be more powerful...
Keep in mind that pinacol is unstable in acidic media and suffers transposition...so direct esterification is a no go!
Maybe substitution of t-butyl chloride or pinacoldichloride with Ag-OClO3 in an adequate solvent to prevent elimination what is a strong competitive
reaction of tertiary alcools...
Addition of HOClO3 on (CH3)2C=CH2 might be a solution for the first step!
If you ever attempt any perchloric ester...beware that it is always a serious risk...so never make it in big quantities...keep it small (<2g) and
far form any glasware, metalware or hard plasticware...
[Edited on 28-11-2011 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  |
but ternary alcohol allow better stability of the hypochlorous ester. I think that perchloric esters must have the same kind of stability problems.
Maybe tertiary alcohol would help entrap the power of perchloric esters in a safer way...
|
Interesting theory, you might be on to a good idea. However, the reasons for instability of perchlorate esters may likely be different from
hypochlorite esters. Perchlorate groups are often much less oxidizing than hypochlorite. Other than the fact that perchlorate esters are
alkylating agents (significantly more so than the nitrate esters), there does not seem to be any literature about the specific chemistry. The fact
that perchlorate esters even exist shows that, despite alkyl groups being less electropositive, the perchlorate group is rendered much less reactive
than >72% conc HClO4, which is known to attack alkanes. Also, chemical stability is different from sensitivity, although there is
some overlap.
Quote: Originally posted by PHILOU Zrealone  |
I have the idea that (CH3)3C-OClO3 must be a first step...trial
then pinacol diperchlorate O3ClO-C(CH3)2-C(CH3)2-OClO3 what must be denser and must display a better OB, thus be more powerful...
|
Another exotic thought would be a perchlorate ester of cubane, with only two perchlorate groups at opposite corners.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Anders Hoveland wrote:
"Another exotic thought would be a perchlorate ester of cubane, with only two perchlorate groups at opposite corners. "
Yes that's it such corners if they hold alcohol would be ternary alcohols...
The best would be cubane-tetrole-tetraperchlorate...with a perfect OB!
C8H4(OClO3)4 --> 8CO2(g) + 4HCl(g)
For the rest about nine post ago, in the last post of Rosco in this tread, there is a link to a very interesting document about perchlorate by
Schumacher.
On page 63 (79 on 280) they speak about the reaction of perchloryl fluoride with ammonia (liquid or diluted)
F-ClO3 + NH3 --> NH4F + NH4NHClO3 (ammonium perchlorylamide)
They also mention K2N-ClO3 and KNH-ClO3
It is very interesting because those are brothers of nitramides and one might expect that if ammonia is replaced by organic amines then it is an open
door to nitramineslike perchloryl compounds
With CH3-NH2 --> CH3-NH2.CH3-NH-ClO3 --> CH3-NH-ClO3
With H2N-CH2-CH2-NH2 --> H2N-CH2-CH2-NH2.O3Cl-NH-CH2-CH2-NH-ClO3 --> O3Cl-NH-CH2-CH2-NH-ClO3
With (CH3)2NH --> (CH3)2N-ClO3
Maybe by interaction of NH4NH-ClO3 (NH3.NH2-ClO3) with CH2=O a triperchloryl variant of RDX (-CH2-N(-ClO3)-)3 would result? Just as NH2-NO2 by
reacting with CH2=O yields RDX...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Quote: Originally posted by PHILOU Zrealone  |
Maybe by interaction of NH4NH-ClO3 (NH3.NH2-ClO3) with CH2=O a triperchloryl variant of RDX (-CH2-N(-ClO3)-)3 would result? Just as NH2-NO2 by
reacting with CH2=O yields RDX...
|
What an interesting idea. Shame, that org. perchlorates are so unstable 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Adas  | Quote: Originally posted by PHILOU Zrealone  |
Maybe by interaction of NH4NH-ClO3 (NH3.NH2-ClO3) with CH2=O a triperchloryl variant of RDX (-CH2-N(-ClO3)-)3 would result? Just as NH2-NO2 by
reacting with CH2=O yields RDX...
|
What an interesting idea. Shame, that org. perchlorates are so unstable 
|
Maybe not all organic perchlorates and that is the point to elucidate here...note that they mention (in the same document) that K2N-ClO3, when dry, to
be highly explosive, sensitive to flame, friction and shock and ...
melting above 300°C what is quite a high temperature for a sensitive compound...
[Edited on 29-11-2011 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Not all organic perchlorates are unstable like the esters. It could be interesting to see what are the properties of a mixture of organic amine
perchlorates which are relatively stable and nonhygroscopic, particularly to see if any ternary or quaternary eutectics have low melting points. Some
candidate materials for such mixtures would be guanidine perchlorate, methylamine perchlorate, trimethylamine perchlorate, tetramethylammonium
perchlorate, betaine perchlorate, choline perchlorate and choline nitratoperchlorate. There are others but these few first come to mind. Hygroscopic
perchlorates like urea perchlorate and possibly glycine perchlorate and dimethylamine perchlorate could make powerful aqueous or alcohol based OB
slurries with ammonium perchlorate which could be interesting compositions.
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
perchlorylamide
With ammonia, FClO3 forms a perchlorylamide: FClO3 + NH3 = ClO3(NH2) + HF. This has acidic protons and they are replaceable by metal ions: K[ClO3(NH)]
and K2[ClO3N] these are colorless, up to 300 C stable compounds, which explode by impact.
Perchloryl fluoride (ClO3F) reacts with aqueous or liquid anhydrous NH3 to form a mixture of NH4F and NH4NHClO3. Ammonolysis of ClO3F in liquid NH3 is
greatly accelerated by traces of NaNH2 (base catalysis).
From an aqueous solution of NH4F + NH4NHClO3, concentrated aqueous or alcoholic solutions of caesium or potassium hydroxides precipitate salts of the
types K2NClO3 and KNHClO3. These salts are highly explosive, and when dry are very sensitive to flame, shock and friction.
Information about ClO3F
Perchloryl fluoride (FClO3) is very stable, poisonous and reactive (Bp. -46.7C, Mp. -147.7C). Electrolysis of saturated NaClO4 in anhydrous HF yields
the compound. Another way in 85-90% yield, is to warm a mixture of KClO4, HF and SbF5 at 40-50 C (Kirk Othmer). FClO3 is also stable up to 400 C, and
hydrolyzes slowly. Grease and rubber tubing has caused explosions.
FClO3 is also made by reacting fluorine with KClO3 at -20 C in SbF5: KClO3 + F2 => KF + FClO3 Or by reacting KClO4 with HSO3F: KClO4 + HSO3F =>
FClO3 + KHSO4 (From: Lehrbuch der anorganischen Chemie by A.F. Holleman, E.Wiberg, N.Wiberg).
| Quote: |
Electrophilic substitution in presence of Lewis acids of FClO3 on aromatics has yielded some interesting compounds via introduction of -ClO3 groups.
Perchlorylbenzene, nitroperchlorylbenzene, etc. preparation is in US3067211, US3937627. Nitrogen heterocyclics also, US3332955 describes
N-perchlorylpiperidine from ClO3F, this compound has been known to explode on storage. The same have been made from Cl2O7 and cyclic nitrogen amines
in an inert solvent. Perchloryl aromatics are usually shock sensitive. 3-Nitroperchlorylbenzene is about as shock sensitive as lead azide, and said to
have a very high detonation rate. Not exactly inert either, even to one of the chemicals used to make them: a perchlorylbenzene/AlCl3 mixture does
nothing, then explodes after some time. With FClO3, alcohols said to turn into extremely explosive alkyl perchlorates.
|
| Quote: |
Dichlorine heptoxide in carbon tetrachloride reacts as a perchlorylating agent with secondary and primary amines. Piperidine, dimethylamine, and
2-ethylaziridine gave N-perchlorylpiperidine, N-perchloryldiethylamine, and N-perchloryl-2-ethylaziridine, respectively. Propylamine and
tert-butylamine gave N-perchlorylpropylamine and N-perchloryl-tert-butylamine. The primary perchlorylamines are acidic and form sodium salts with
aqueous sodium hydroxide. Yields ranged between 60-83%.
|
"Reactions of dichlorine heptoxide with Amines"
Charles D. Beard, Kurt Baum
N-perchloryl organic compounds are apparently extremely sensitive, prone to explode for little reason:
| Quote: |
N-perchlorylpiperidine is a dangerously sensitive material. It explodes on heating and on contact with anhydrous piperidine. A sample of the
oil exploded with violence in an outside bunker as a possible result or exposure to heat or the sun...
|
The ClO3F route to organic perchloryl compounds gives very poor yields, so the Cl2O7 route, which gives very good yields, may be preferable. Each
route, however, presents its own unique safety considerations; whereas working with ClO3F has similar hazards to working with fluorine (although
supposedly glass shows good resistance to ClO3F), working with Cl2O7 presents a severe explosion hazard. As for dissolving Cl2O7 in CCl4, probably
best to use a large excess of CCl4, and to mix immediately before use since there will likely be a spontaneous react after a short time. Preparation
of solutions of Cl2O7 in CCl4 is also described in: F. Meyer, Keszler (Ber. 54, [1921] 569). It almost seems that anhydrous HClO4 is more reactive
towards CCl4 than Cl2O7, which is unexpected since Cl2O7 is the "acid anhydride" of HClO4. HClO4 is insoluble in CCl4, and gives upon shaking, a green
emulsion, which discolors brown after several minutes welling up under formation of HCl and COCl2 (Vorländer, v. Schilling, Lieb. Ann. 310 [1900]
374).
[Edited on 30-11-2011 by AndersHoveland]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Below are the structures for N-perchlorylpiperidine, N-perchloryldimethylamine, N-perchlorylpropylamine.
One of the likely resonance structures for N-perchloryl compounds that would explain their relative stability (at least the fact that they do not
immediately explode) is shown in "figure R".
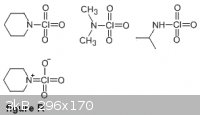
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Organic Perchlorate Esters
http://www.dtic.mil/dtic/tr/fulltext/u2/271592.pdf
Organic Perchlorates
http://www.dtic.mil/dtic/tr/fulltext/u2/902549.pdf
Advanced Oxidizer Research 1964 I
http://www.dtic.mil/dtic/tr/fulltext/u2/352713.pdf
Advanced Oxidizer Research 1964 II
http://www.dtic.mil/dtic/tr/fulltext/u2/361116.pdf
Advanced Oxidizer Research 1966
http://www.dtic.mil/dtic/tr/fulltext/u2/376350.pdf
Research in Fluoro-Nitro Compounds
http://www.dtic.mil/dtic/tr/fulltext/u2/347414.pdf
Related Post _
http://www.sciencemadness.org/talk/viewthread.php?tid=1778&a...
[Edited on 20-12-2011 by franklyn]
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Ethylene diperchlorate? I think it can be made by reacting Cl2O6 with ethylene.
EDIT: Not possible, since Cl2O6 consists of [ClO2]<sup>+</sup> [ClO4]<sup>-</sup>
[Edited on 6-5-2012 by Adas]
Rest In Pieces!
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
"Diethyl ether detonated when it was added to perchloric acid"
Chemical Risk Analysis: A Practical Handbook, Bernard Martel, 267
There might be a chance it could be possible to prepare some ethyl perchlorate by cautiously adding 70% perchloric acid into diethyl ether. I really
do not know what would happen, it might be worth investigating, though of course it should be treated as if it could detonate at any moment at all
times.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
well anders, they dont describe what concentration, it could of been 99% in which also goes off with a nice snap with paper added (:
ether does form peroxides just with air, i suppose not even anhydrous HClO4 would be needed for it..?
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Well if PHILOU is right, then why stray to such incongruities as cubanes? Tetrabromoadamantane and adamantanetetrol are readily prepared. Perfect
balance too. It's probably a dreadful notion but who knows.
Nobody linked this either- http://www.dtic.mil/dtic/tr/fulltext/u2/754778.pdf
[Edited on 8-8-2013 by halogen]
|
|
|
Davin
Harmless

Posts: 36
Registered: 5-12-2012
Member Is Offline
Mood: No Mood
|
|
The original files for the synthesis of ethyl perchlorate on Blaster's site are 404'd. Could someone re-upload them?
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
i found two method for the preparation of ethyl perchlorate:
the first one see [1], uses ethyl iodide with lithium perchlorate in aprotic solvent (Et2O, AcOEt...) in the presence of an oxidizer
(Cl2, m-CPBA, H5IO6).
the second paper [2], uses ethyl iodide and silver perchlorate in equivalent amount (see page 23 for the procedure from [2]) to form ethyl perchlorate
in absolute ether.
also, this is the reference of Julius Meyer & Walter Spormann that Blaster translated to make his ethyl perchlorate.
http://onlinelibrary.wiley.com/doi/10.1002/zaac.19362280404/...
Dany.
Attachment: [1].pdf (67kB)
This file has been downloaded 668 times
Attachment: [2].pdf (1.6MB)
This file has been downloaded 771 times
[Edited on 12-1-2014 by Dany]
|
|
|
| Pages:
1
..
4
5
6
7 |