| Pages:
1
..
57
58
59
60 |
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
2x 500ml Butyric acid repackaged from HDPE to 1L glass bottle.
The place stinks, I feel like going out for a walk...
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Trash away the old plastic bottles. I repackaged butyric acid from plastic to glass few years ago and put the bottle outside of house as I was curious
how long it will last to lose the stinky smell... it lasted for few months for butyric acid and more than 1 year for valeric acid. Plastic is good for
transport (glass is fragile) but for storage you did well with repackaging into glass. Storage of phenylacetic acid the same - very bad stinky scent
in whole house when it was in original plastic container... problem disappeared when stored in glass.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I purchased material for making a good quantity of bromine very cheaply:
- 1 lb of KBrO3, see here: https://www.ebay.nl/itm/125184055068
- very cheap 48...49% HBr from here: https://www.laboratoriumdiscounter.nl/nl/waterstofbromide.ht...
Especially the latter is a really good price. I received my order with the HBr from laboratoriumdiscounter, and it is a high quality product. It is
nearly colorless, which is quite unique for HBr. Usually, the stuff is yellow, piss-colored, but this only has a very weak yellow color.
I intend to carefully mix the KBrO3 and HBr, making Br2, without any other stuff in it (especially no chlorine, which may lead to BrCl-impurity). The
Br2 then can easily be distilled off.
Making Br2 is very simple with this. I'll mix appr. 250 grams of KBrO3 with the liter of HBr, which will produce Br2 and leaves a slight excess of HBr
in solution. The Br2 can be distilled off into a little concentrated H2SO4, which makes it dry. The Br2 then again is distilled off from the H2SO4,
leaving pure and dry Br2. In this way, I will have appr. 700 grams of pure bromine, which is around 230 ml of bromine.
The liquid from which Br2 initially is distilled will have KBr (and a little HBr and probably also some remains of Br2, bound as Br3(-)). This liquid
can be boiled down to drive off the last remains of HBr and Br2 and leave 170 to 180 grams of KBr behind, which also is useful.
|
|
|
ManyInterests
National Hazard
   
Posts: 966
Registered: 19-5-2019
Member Is Offline
|
|
I've ordered potassium chloride (because I can't get any from sodium free salt lately), Resorcinol, and 28% ammonium hydroxide. While I do want to
make my own ammonium hydroxide, I want some ready before I make my first synthesis.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Warning: Do not buy the KBrO3 from that first seller. It is fake. It is not KBrO3. I think it simply is expensive KBr. It dissolves very easily in
water and when a drop of aqueous HClO4 is added, you get a white precipitate (must be KClO4), and when a strong oxidizer is added to the acidified
solution, then Br2 is produced. I asked either a refund, or a shipment of real KBrO3. The seller seems to be chinese. The object location is in
Bulgaria, but probably the material is shipped from some warehouse, which is done more and more by chinese sellers.
The HBr from the second link is good stuff, that company definitely is trustworthy.
I get the impression that buying things from eBay has become more troublesome the last two years or so. I already buy from eBay from 2005 or so, many
chemicals, and many electronics parts, but my last three purchases all were fake or crap (one crap electronic device, an ozonizer, which was totally
bogus, crappy Cu(OH)2, which was anything but Cu(OH)2, and now this failure with KBrO3). All items were from chinese sellers, but in the past, I also
purchased many things from chinese sellers and always had good, or at least decent experiences. But things have changed . . .
Edit(woelen): I was offered a refund by the seller, or a reshipping of another parcel. I chose a refund. After accepting the offer of a refund, I
received it just a few hours later. So, at least the seller recognized the error and did his best to keep me happy.
[Edited on 14-5-22 by woelen]
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
That's a bummer. I guess you could always go the oxidizer+sulfuric acid+KBr method if you're stuck with that stuff. I like to do that with hydrogen
peroxide as the oxidizer since it's cheap (for me) and clean, though I know it's harder to get in Europe, so probably not a viable option for you.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
It's really strange all the bogus Ebay products.
Don't know if other countries are different but here in the US a bad Ebay products can be returned within a certain time period with the seller
forced to pay the shipping for return. (Or at least could a couple of years ago. I quit selling there because they kept changing their rules to my
disadvantage)
This even happens with good products if the buyer insists they are bad. Sometimes the generous policy is used to screw the seller, but I found this
to be very rare in my hundreds of ebay sales. Most people are honest, even if they're buying stuff I'm pretty sure is being used for illegal purposes.
If anything the shady buyers were more honest.
Anyway, with this policy selling bogus products only pays off if the seller doesn't complain to Ebay before the time window for complaining is up. I
find it surprising that this happens enough to pay off for the crooked seller.
Maybe the seller you bought from got screwed by his source? Testing your wares is problematic unless you're buying bulk (or very conscientious).
I myself wound up with egg on my face a few times when I sold second hand chemicals that must have been improperly stored and had deteriorated.
(Some protease inhibitors and a couple of items that can't tolerate California summers unless in a refrigerator at least.)
I tested what I could; but if it's a closed container you hate to open it for testing if opening it may lead to degradation or contamination.
I finally disposed of a number of valuable but 'iffy' used chems as I just couldn't stand the possibility that I might screw up somebody's reaction
that they put a lot of effort and expense into because I was unknowingly peddling junk.
|
|
|
Keras
International Hazard
    
Posts: 1013
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by woelen  |
All items were from chinese sellers, but in the past, I also purchased many things from chinese sellers and always had good, or at least decent
experiences. But things have changed . . .
|
IMHO, you just had a stroke of luck. I shun Chinese products like hell. And it’s getting worse with the Covid.
Laboratorium Discounter is great, though. They’re a bit—how to put it mildly—messy in their organisation: their lead times are usually way too
optimistic, especially for compounds they don’t stock like phosphorus pentoxide, and I once ordered calcium hypochlorite and got sodium nitrate
instead! :p—but they’re otherwise very fair priced and reliable.
As for making bromine, I’m surprised you don’t use a simpler method. I just tried KI + sodium percarbonate. For some reason, it didn't work. It
must probably be carried out in an acidic solution. I’ll redo the experiment later. However, I substituted sodium percarbonate for Oxone, and it
instantly oxidised I⁻ into solid I₂, so I think you can obtain Br₂ very neatly by dropping an Oxone solution into a KBr solution.
[Edited on 14-5-2022 by Keras]
|
|
|
Texium
Administrator
       
Posts: 4665
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: Preparing to defend myself (academically)
|
|
I would
expect so. It would be very similar to the sulfuric acid + hydrogen peroxide method.
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I know other methods of making Br2, but with cheaply available HBr and cheaply available KBrO3 things get really easy. Of course, I can use other
oxidizers, but first I'll see whether I get another shipment from that chinese seller (or get a refund).
|
|
|
Keras
International Hazard
    
Posts: 1013
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Well, Oxone is basically a Caro’s acid salt, so yes, that’s the same.
Oxone is cheap too. I don’t remember how much I paid for it but it was quite low.
What I don’t understand is why sodium percarbonate didn’t work. I added a very few crystals of TsOH to the mix, and sure the brown colour appeared
immediately, but then after a swirl or two the mixture became clear again. I’ll retry using citric acid to neutralise the sodium carbonate.
|
|
|
Keras
International Hazard
    
Posts: 1013
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
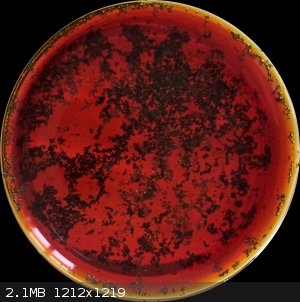
Okay, so I redid the experiment with sodium percarbonate using citric acid. Dissolved a few milligrams of KI into 2 mL or so of water, added a few
milligrams of citric acid. When everything had dissolved, added small portions of sodium percarbonate → solution became yellow then brown then with
added KI a lot of I₂ crystals formed. So this procedure works with iodine, and probably with bromine too, provided you operate in acidic solution
(strong acid not needed).
[Edited on 15-5-2022 by Keras]
|
|
|
Tsjerk
International Hazard
    
Posts: 3035
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Doesn't citric acid react with iodine/bromine?
|
|
|
Keras
International Hazard
    
Posts: 1013
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Apparently not? I didn't assay the crystals, though, but they seem to be perfect iodine at first sight.
|
|
|
Boffis
International Hazard
    
Posts: 1897
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Keras  |
Apparently not? I didn't assay the crystals, though, but they seem to be perfect iodine at first sight. |
Oh yes it does, the product is pentabromoacetone which is produced almost quantitatively and is used as a means of analysis for citric acid. I have
some papers somewhere on the reaction if you are interested.
|
|
|
Keras
International Hazard
    
Posts: 1013
Registered: 20-8-2018
Location: (48, 2)
Member Is Offline
|
|
Quote: Originally posted by Boffis  |
Oh yes it does, the product is pentabromoacetone which is produced almost quantitatively and is used as a means of analysis for citric acid. I have
some papers somewhere on the reaction if you are interested. |
Yes, please 
This document (p.3, first §) suggests otherwise, though.
[Edited on 15-5-2022 by Keras]
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I purchased 1 liter of 30% NaClO3 (pure, no NaCl in the mix, just water). This is within the EU-rules and sale of such solutions is allowed. I intend
to try making concentrated solution of NaClO4 with this. I have a PbO2 electrode and do not want to spend that on making NaClO3 from NaCl (this
corrodes the anode more than I like) and with this NaClO3-offer I just can buy this liquid, stick in the electrode and make NaClO4. The solution of
NaClO4 then can be mixed with conc. HCl to make quite pure HClO4 (at least good enough for experiments in making salts of transition metal complexes).
It's good to see that there are suppliers who are willing to sell stuff like NaClO3 to private individuals in such a way that it is inside the
EU-regulations and on the other hand is easy to purify and to use in experiments.
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Keras  |
Laboratorium Discounter is great, though. They’re a bit—how to put it mildly—messy in their organisation: their lead times are usually way too
optimistic, especially for compounds they don’t stock like phosphorus pentoxide, and I once ordered calcium hypochlorite and got sodium nitrate
instead! :p—but they’re otherwise very fair priced and reliable.
|
I placed an order on the first of June having that in mind and called them to say hello today as the order was supposed to be dispatched in 3-4 days.
Apparently it's going out tomorrow so I can confirm what you said in a very polite way. They are a bit messy 
Joke aside, they seem to have several warehouses. The chemicals were not the problem but the glassware they had at another place.
I'm surprised I had to go out of my country to find pre-made ampoules but I cant wait for them to arrive. There are so many things I'd like to weight
and seal in ampoules for display or for pre-weighting compounds / reagents. This totally outweighted the cost of the ampoules.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
DocX
Hazard to Others
  
Posts: 179
Registered: 22-10-2015
Member Is Offline
Mood: No Mood
|
|
A bunch of general purpose solvents (DCM, DMF, GAA), a bit of nice polyphosphoric acid (to have an alternative to acetic anhydride), some
cyclohexylamine to aminate stuff and a pinch of thiourea for ring closure. From the ultra-professional Chemship1978.
I will try my hands on thalidomide synthesis this summer. Why? Because now I can!

[Edited on 20222222/7/6 by DocX]
|
|
|
woelen
Super Administrator
        
Posts: 8080
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I purchased a few kilos of boric acid, now that I still can. I see that more and more suppliers do not sell boron oxide, boric acid and borax to
private persons anymore. Other suppliers ask paperwork. These boron compounds are not that interesting, but it is good to have some boric acid around
and have the option to make borax or boron oxide.
Boron compounds in oxidation state +3 have very low acute toxicity, but they have adverse effects on the unborn child, especially, when there is
frequent exposure to small doses (e.g. when a pregnant woman uses boron-compounds frequently in the household). For that reason, boric acid and
derived products are made inaccessible for the general public more and more and many companies only sell these to professional users (e.g.
laboratoriumdiscounter.nl does not sell it to private persons, and deoplosmiddelspecialist.nl still sells it, but requires paperwork).
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
2lbs of Sodium Nitrite from Duda Diesel.
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Just restocked with solvents.
4 L anhydrous ethanol
1 L isopropyl alcohol
1 L methanol
1 L acetone
1 L DCM
All from Sydney Solvents, very happy with price, packaging and delivery time.
|
|
|
charley1957
Hazard to Others
  
Posts: 173
Registered: 18-2-2012
Location: Texas
Member Is Offline
Mood: Winter’s winding down. Yay!!
|
|
I hate to admit this but I have become a chemical hoarder. Things are disappearing both from eBay and local shelves. For example, I was very lucky to
find three gallons of DCM-based paint stripper at an ACE hardware store. The cans were rusty and dusty, had been on the shelf for years. I’ve
searched high and low for more and there is no more to be found. I recently bought a keg of 98% sulfuric acid for $160 from an oilfield chemical
supply house. Things like sodium bromide I can’t get off the shelf anymore at the pool section of any store. I’ve never been able to find ammonium
nitrate at any garden supply section. eBay only sells potassium metal in tiny little amounts for exorbitant prices. Even potassium iodide has gotten
way expensive. I’ve had iodine crystals on order since January from China and I suspect customs will never let them through as I’ve ordered three
times now. Who would have thought that some of these things would become so scarce or expensive? And what’s going to become prohibitively expensive
or extinct next? Does anyone know of a source of potassium metal that won’t break the bank? I really hate buying in such large amounts when I can
find it but I’m afraid if I don’t I’ll be without before long with no prospects of getting anymore of some things. I can still get things OTC
like hydrochloric acid, toluene, acetone, lye, copper sulfate, etc but the prices are going up and up and who knows when they’ll just disappear
permanently from the shelves. I know all of us are in the same boat and it’s a changing world and you just can’t get some things now that were
once easy to get and cheap. Anyway, just a rant as much as anything, but I sure hate being a hoarder.
You can’t claim you drank all day if you didn’t start early in the morning.
|
|
|
Gammatron
Hazard to Others
  
Posts: 125
Registered: 30-8-2022
Location: Abandoned Uranium Mines
Member Is Offline
|
|
I ditto that about hoarding chemicals. I have gallons of H2SO4 and 27 & 40% H2O2 and several kilos of nitrates for that same reason.
Seattle Pottery Supply has really good prices, also fireworks cookbook has the best prices and availability I've seen for things like metal powders
and oxidizers.

|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
1.062 grams of Osmium.
Really quite interesting to handle and compare when you have 1 gram quantities of W / Au. Lead even feels light 
My collection of available and storable elements will almost be complete when I place my next order with Onyxmet.
Main focus now: 1 gram Iridium before it becomes as unavailable as Rhodium.
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
| Pages:
1
..
57
58
59
60 |