| Pages:
1
..
3
4
5
6
7
..
15 |
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Isn't science wonderful (better living through chemistry).
I'm glad to hear that you are doing well. In my limited experience (16-yr cancer survivor), attitude is everything, and you seem to have the right
one!  I became much more relaxed when I finally realized that there was no point
in obsessing over things that I had no control over. I became much more relaxed when I finally realized that there was no point
in obsessing over things that I had no control over.
Regard, Charlie
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
dehydrated some oxalic acid under toluene. It would have been a lot easier to have a Dean-Stark but otherwise it went well
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Got some orange peels steam-distilling, no broken glassware yet!
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Herr Haber
International Hazard
    
Posts: 1236
Registered: 29-1-2016
Member Is Offline
Mood: No Mood
|
|
I routinely check on the chemicals I have in store.
Some of them are in cold storage for a variety of reason.
What a surprise to be greeted by a strong ammonia smell when I looked at the box containing solid bases (lots of NaOH, KOH even more NaHCO3 etc.).
Culprit was 1 kg of food grade Ammonium bicarbonate. Who would have guessed the decomposition temperature was so low !
The spirit of adventure was upon me. Having nitric acid and copper, I had only to learn what the words 'act upon' meant. - Ira Remsen
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Finally completed Potassium Azide synth. What a pain that was. Making the Hydrazine Sulfate was the easy part surprisingly.
I started by making fresh isopropyl nitrite with some old homemade nitrite of dubious quality and of course it failed. Made a new good looking triple
size batch which i accidentally fused into the bottom of the beaker. I lazily decided i would not need to dissolve it again because it is so soluble
but it ended up getting coated with Potassium Chloride and it failed again. New batch again, takes a day to boil down all the while my hotplate keeps
failing triggering the fuse of the apartment repeatedly. Because i poked a hole in my wide steel container i have to use a taller one than ideal for
the batch making it extremely difficult to get the water out. I had to use up all the fuel of my two burners. After two days work i get my isopropyl
nitrite.
Azide synth goes really well until the ***************** hotplate fails again. Luckily it worked out anyway though my product is an embarassingly
yellow moist powder. In the end i think i used too much nitrite. At least now i can go to uni with my mind at peace having got my fill of chemistry
for some time.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by Σldritch  | Finally completed Potassium Azide synth. What a pain that was. Making the Hydrazine Sulfate was the easy part surprisingly.
I started by making fresh isopropyl nitrite with some old homemade nitrite of dubious quality and of course it failed. Made a new good looking triple
size batch which i accidentally fused into the bottom of the beaker. I lazily decided i would not need to dissolve it again because it is so soluble
but it ended up getting coated with Potassium Chloride and it failed again. New batch again, takes a day to boil down all the while my hotplate keeps
failing triggering the fuse of the apartment repeatedly. Because i poked a hole in my wide steel container i have to use a taller one than ideal for
the batch making it extremely difficult to get the water out. I had to use up all the fuel of my two burners. After two days work i get my isopropyl
nitrite.
Azide synth goes really well until the ***************** hotplate fails again. Luckily it worked out anyway though my product is an embarassingly
yellow moist powder. In the end i think i used too much nitrite. At least now i can go to uni with my mind at peace having got my fill of chemistry
for some time. |
tine to get a better hotplate :')
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Currently dissolving some copper wire in dilute perchloric acid + peroxide 
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Am experimenting with boric acid catalysts in Fischer esterifications, I have seen too many people mention how it may work and haven’t seen anyone
try it.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
John paul III
Hazard to Others
  
Posts: 110
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
Made some calcium peroxide from calcium acetate and 3% H2O2 to see if it worked. Will upscale with 60% H2O2 I left in my rental apartment and see what
this thing does mixed with sugar
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
mayko you certainly had / have / will have interesting ideas which I'm missing due to my limits, but why didn't you react CuO + HClO4?
Did you want to prepare Cu(ClO4)2 ASAP and avoid waste of time to prepare/buy CuO?
Was it an attempt to prepare very pure product (metallic Cu of high purity is more available than similar grade purity CuO)?
Or did you want to dissolve Cu in anything else than already well known, boring, stinking ways HNO3, hot conc. H2SO4? HCl + H2O2 would stink too so
that's why you used HClO4? Also Na2S2O8 is used for dissolving Cu layer of PCB for electronics and another etching reagent for Cu PCB is FeCl3.
|
|
|
j_sum1
Administrator
       
Posts: 6372
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I did an ethanol extraction of some camphor laurel leaves. This was more an excuse to use the soxhlet than anything else. But given that my street is
lined with these trees, camphor extraction has been on the list for a while. I doubt my efficacy. I seem to have a lot of chlorophyll even though the
leaves are dry and brittle. I will leave my bag of leaves a month or two more and try again. Probably a different method too.
Today I got my 8y old son to help me distill and recover the ethanol. It was a chance to use my Graham condenser: the cool appearance surpasses its
functionality in most situations but it performed beautifully in this instance. Distilling ethanol is so satisfying: it just pours out of the
condenser. My son enjoyed it too. Good times.
|
|
|
Fery
International Hazard
    
Posts: 1052
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
j_sum1 - steam distillation?
as you have plenty of material for free, maybe you can build some medium scale apparatus from an old pressure cooker
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Quote: Originally posted by j_sum1  | | It was a chance to use my Graham condenser: the cool appearance surpasses its functionality in most situations but it performed beautifully in this
instance. Distilling ethanol is so satisfying: it just pours out of the condenser. |
Yup. Only use i've ever had for my Graham. LOL
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Well for all of those who have wondered, boric acid does not catalyze fischer esterification alone. Ill add a dehydrating agent to see if that can
drive it away from hydrolysis.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Tsjerk
International Hazard
    
Posts: 3035
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
I have been looking around on the webs today a bit after finding some publication describing use strong acid ion exchange resin as a catalyst for
esterfications...
After being done you only have to filter and the catalyst is gone and reusable.
Appearantly any old "Brita" kitchen drinking water purifier can be opened to get the resin which can be regenerated by soaking in acid.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Really? I thought the Brita filters only had activated charcoal.....
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Tsjerk
International Hazard
    
Posts: 3035
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
At least not the Dutch ones.. I haven't taken one apart yet but the brand Brita cartridges should have a resin compartment next to the charcoal
compartment.
Edit: as long as it states to de-harden water it should have the sulfonated styrene resin.
[Edited on 3-1-2020 by Tsjerk]
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by Tsjerk  | At least not the Dutch ones.. I haven't taken one apart yet but the brand Brita cartridges should have a resin compartment next to the charcoal
compartment.
Edit: as long as it states to de-harden water it should have the sulfonated styrene resin.
[Edited on 3-1-2020 by Tsjerk] |
That sounds awesome, I have one I can use!
EDIT:
Heres the box, it contains both activated carbon and ion exchange resin, which I assume is a sulfunated styrene.
In a bit I will take it apart and attempt an esterification with it. I have never tried using a sulfunated resin but I have heard they are excellent
catalysts.
[Edited on 1-3-20 by Abromination]
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Sorry for the double post, wouldnt let me include pic
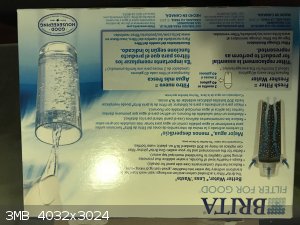 
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Isopropyl nitrite. Per Rhodium.

*edit* photos of yield
 
[Edited on 1-20-2020 by arkoma]
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
DraconicAcid
International Hazard
    
Posts: 4412
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
We happened to get some dry ice in with a sample the other day, so I did a Grignard reaction with benzyl chloride to get phenylacetic acid. I messed
it up, but still managed to get a small yield of white, clean-looking product. I've washed my hands a dozen times, but I can still smell it.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I ran out of concentrated ammonia solution a while back and I've had some crappy cold-pack ammonium nitrate languishing on the shelf, so I'm
liberating the gas with lye and redissolving. The sodium nitrate will go back on the shelf until I run out of nitric acid. I was surprised at just how
much NH4NO3 it took (~360 g) to make so little ammonium hydroxide (~250 mL)!
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Do you know the purities of your starting materials? My calculations (always iffy), show that 4 mols of ammonium nitrate should yield 4 mols of
ammonium hydroxide. 4 mols/250 mL = 16 M which seems to be high. Did you titrate the ammonium hydroxide solution to determine its molarity?
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I based my calcs off this passage from Wikipedia:
| Quote: |
At 15.6 °C (60.1 °F), the density of a saturated solution is 0.88 g/ml and contains 35.6% ammonia by mass, 308 grams of ammonia per litre of
solution, and has a molarity of approximately 18 mol/L. At higher temperatures, the molarity of the saturated solution decreases and the density
increases.
|
So it looks like your math is as good as the starting numbers were! I haven't titrated, but most of the expected mass increase is there, and I haven't
had the huge cloud of excess ammonia gas I'd expect if there was too little water. Apparently ammonia is just ludicrously soluble! 
Attachment: ammonia_concentration.xlsx (5kB)
This file has been downloaded 541 times
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
quote: So it looks like your math is as good as the starting numbers were!
What do you know! Apparently it is true that a blind hog finds an acorn every now and then! Obviously I should have checked the solubility of ammonia
in water. I suppose an accurate pH measurement of the solution would allow a calculation of the concentration also. I am very gratified to see
someone report a result that they have checked against theory. This doesn't seem to be the case as often as it should be, and I confess that I am
guilty of this too often.
|
|
|
| Pages:
1
..
3
4
5
6
7
..
15 |