| Pages:
1
..
3
4
5
6
7
..
13 |
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
BCDMH is definately an interesting chemical and a darn hard one to figure out how to reduce.
The only literature on it, is what is provided by the pool and spa industry which is generally written for "those not skilled in the art".
The only practical way I can think of reducing BCDMH is by adding NaOH and heating then reducing the formed halogen compounds by adding sulfuric
acid... which may or may not work.
When BCDMH is placed into water, it dissolves into hypohalous acids and hypohalic ions whose equilibrium is based upon temperature.
The addition of NaOH neutralizes the acids formed and turns them into hypohalites.
Adding heat causes the BCDMH to dissolve more readily and the hypohalite salts to undergo an auto-oxidation-decomposition into halides and halates.
The reaction would be:
BCDMH + 2 NaOH = 1 NaBr + 1 NaCl + 1 NaBrO + 1 NaClO
1 NaBrO + 1 NaClO ---- heat ---> 2 NaBr + 1 NaBrO3 + 2 NaCl + 1 NaClO3
(X represents a halogen)
6NaX + 2 NaXO3 + 3 H2SO4 = 4Br
I know the equation is unbalanced but it should liberate most of the available bromine due to the chlorine liberated at the same time as the
bromine... the free chlorine would undergoes substitution reactions with the bromo compounds.
It is unknown whether or not the NaOH or Sulfuric acid would react with the spent DMH.
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
How about just bubbling chlorine into an aqueous suspension of BCDMH?
This should result in formation of dichlorodimethylhydantoin (the Br on the BCDMH gets replaced with Cl, due to higher reactivity of Cl) and
quantitative liberation of bromine. It can be distilled out of the mix (which will have to be kept cold during Cl2 addition).
However, the bromine such obtained will contain some chlorine, bound up as BrCl and also physically dissolved.
Obtaining pure bromine from this is easy: the crude bromine is stirred with a NaBr solution for a while and then distilled out of it.
This replaces all the Cl atoms in the crude bromine with Br, again due to higher reactivity of Cl.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
The problem is that BCDMH is not very water soluble...
And, as soon as it does dissolve you end up with HXO molecules.... which may or may not be of any consequence.
I landed 3 pounds of BCDMH for $10 this afternoon at Lowes on clearance (old stock from last year) and 12 oz of NaBr for another $10 (pocketbook still
hurting).
Tonight I plan on doing a little expirimenting... will post the results later on...
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Interesting results... just for shits and giggles I decided to chunk a pellet into some 10 molar HCl.
Immediatly a vigorous reaction took place and it fizzed like crazy releasing chlorine gas or perhaps a combination of CL2 and perhaps some
interhalogen BrCl.
As the HCl was used up, the reaction slowed down and the solution began to turn orange... I know that there has to be some bromine in there being
released.
When everything slowed down the reaction turned milky orange
The reaction appears to be non-exothermic... this could be a very very convienient source of bromine or for that matter chlorine if I can just figure
out how to get everything separated!

|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Good thinking with the HCl... what with the common ion effect and such. Try it with a more dilute solution and see if you get better results. The
kind of reaction you describe from the 12 M solution doesn't sound very scale-able.
I don't suppose you could get away with neutralizing the solution with excess NaOH, then just distilling out the bromine?
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
NaOH would react with bromine making hypobromite, and if it was hot enough hypobromite woud decompose yielding bromide and bromate.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
You can't add NaOH to an elemental halogen without making the hypohalite IIRC so that is probably out.
Actually what I really need is a more concentrated solution of HCl... or so the theory goes.
Personally I want the solution to become so saturated that the bromine simply falls out of solution... then it can be mechanically separated then
dried and purified over concentrated H2SO4.
The problem with the setup above is that the Cl2/BrCl "off gases" so fast that any formed Cl2 molecules dont have a chance to react with the
bromine... diluting the HCl might slow down the reaction, but it wouldn't be too good for precipitating bromine.
Tommorow I might try to snag a clear champagne bottle and build a pressure reactor... I figure if I chill the HCl down to nearly freezing the pressure
built up from the reaction will be well within the safety limits of the bottle... as long as the temp stays below 40 degrees the pressure inside the
bottle shouldn't get over 60PSI according to my data.
Trying to contain the reaction will be interesting. I theorize that the the formed chlorine will eventually take over everything and the bromine will
fall out of solution or else the mix will reach equilibrium and stop...
Anyway you go though, a setup like the above would make a great chlorine gas generator as all it would take is a needle valve and a pressure gauge
added onto the stopper.
|
|
|
eesakiwi
Harmless

Posts: 27
Registered: 10-8-2005
Member Is Offline
Mood: drawnout
|
|
Shit, eesakiwi just put pool Bromine (white powder, crushed up a bit) in a tall glass tube.
Poured on some HCL acid, it foamed and spit a bit so swIm poured some Parrifin oil over it & left it.
The HCL: layer kept reacting with the Bromine powder & moved down the tube, as it reacted the reaction face it got orangey, then darker, then
red, then a thicker layer of red.
The Parrifin layer stopped the smell coming outta the tube & still 'floated' above the HCL layer/reaction.
Untill it got to the bottom of the tube & there was a thick layer of Bromine, & its still there.
This was all about 1+ ysr ago.
SwIm'd just put the powder in the tube, pour over it a 15mm layer of Parrifin oil, & then use a syringe to inject the HCL into under the Oil
layer.
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Tonight is the night.
I'm going to attempt a 2 mole reduction and isolation of Br2 using NaBr using H2O2 and H2SO4 which have shown to be very good in tests. All methods
using the BCDMH have been pretty much fruitless. KMnO4/H2SO4 tests have been less than desirable along with any method involving chlorine. The former
suffers from the Br bonding with the oxidant and the latter suffers from equilibrium issues.
Reactants are as follows.
Rooto brand sulfuric acid drain opener
Salon Care 40 volume clear developer (12% H2O2 by volume)
Dupont spa start up/reserve sodium bromide
The setup I'm using involves a 1 liter FB flask, claison adapter, 500ml addition funnel, 20 ml Barret style moisture test reciever with overflow
(cadillac of dean stark traps), 400mm high efficiancy coil reflux condensor.
The idea is to drip the H2SO4 into a mixture of pre-dissolved NaBr in H2O2 similar to what Bromic Acid did on his website the first time around. But,
instead of trying to collect the highly volatile condensed bromine in an ice cooled flask, it will be collected in the barret reciever. This way any
fumes that are re-emitted will simply rise back up into the condensor and be re-condensed preventing vapor losses similar to what Bromic was
experiencing. When its time to empty, the drain cock on the barret is opened and the contents are drained into an ice cold container with PTFE top.
The reaction pilfered off of Bromic's site is as follows:
H2O2(l) + 2NaBr(aq) + H2SO4(l) ---> Br2(g) + 2H2O(l) + Na2SO4(aq)
So when you figure it up, 100ml of 12% H2O2 contains 1/2 a mole of H2O2. So to reduce 2 moles of NaBr, 200ml of H2O2 is required. Since the rooto
causes some catalytic reduction and the solution would be right up near its saturation point, a slight amount more H2O2 will be added, say another
25ml or just enough to get everything dissolved. Not very much H2SO4 is needed, but will be added until the solution is completly acidic.
Well I'm off to hook everything up. Wish me luck. Will take pics.
|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
Good luck! Bromine is some really remarkable stuff, and any time you can make some on your own instead of having to purchase a sample of it is a good
thing. (Shipping costs are kind of nuts. Many carriers don't like shipping the stuff).
I bought a good deal of bromine off of E-Bay a few years ago when you were still able to buy things like elemental bromine, sodium, potassium,
rubidium, phosphorus, etc. from there. The bromine was shipped in a normal amber glass bottle with a PTFE cap. This bottle was in a plastic bag
which was then put into a LARGE metal can surrounded by vermiculite. (The bottle was a 50 mL bottle as my purchase was of 100 grams of Br2). Anyway,
after about a week of having the bromine I noticed that everything in the area the can was in was starting to rust severely. I opened up the can and
saw that the inside was HEAVILY corroded. The Br2 had eaten right through the vial's cap and was starting to leach out!  I called up the person I had purchased it from and they VERY quickly overnighted a
new bottle to store the bromine in. (This was one specially designed to contain corrosive materials, and was basically pure Teflon). I called up the person I had purchased it from and they VERY quickly overnighted a
new bottle to store the bromine in. (This was one specially designed to contain corrosive materials, and was basically pure Teflon).
I will never forget the smell of the bromine as I was transferring it. It reminded me of a skunk that took a bath in concentrated bleach. Nasty
stuff. I made the transfer outside and was happy to be upwind of the stuff. Thankfully, I didn't have any further problems with it. I eventually
got a sample of about 5 mL of it sealed in a glass ampoule for my element collection and traded the rest of it away for other elements I needed.
(Like some Ir and Os metal).
In high school, I also learned first hand that bromine will leach into glassware if given a chance and remain there for MANY years. I was working on
a lab in chemistry class which required some glass tubing to be bent. My teacher had me go into the supply room and get some glass tubing that was in
one of the drawers. Apparently the previous chemistry teacher used it to generate and condense elemental bromine, but my then teacher did not know
that. The glass looked perfectly fine to me so I went and brought it out. My lab partner started to hold the glass in the bunsen burner flame to
begin the bending process when the entire tube turned slightly yellow, then orange, then red, then red-brown. A reddish-orange gas began to eminate
out of it and up towards the ventillation duct in the ceiling. Stupid me was in between the glass and the duct and got a very large lungful of dilute
Br2 vapor. I think I was coughing for about two weeks after that. heh. I was just shocked that the Br2 could get trapped in the glass for such a
long time and not leach out.
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Wow. All I can say is wow.
Bromine vapor is some tough stuff to try and condense! Thank goodness I had my respirator on... fumes were going everywhere.
DO NOT, I REPEAT DO NOT TRY TO DISTILL BROMINE WITHOUT A RESPIRATOR ON, PREFERABLY ONE THAT IS AIR LINE SUPPLIED!
I decided to make some last minute changes, one being to up the batch size to 4 moles (408 grams) and use ACS sulfuric acid.
All in all I managed to catch 153 grams of wet reddish black bromine. I was hoping to get a yield of around 80% but I figure my yield will be about
38-43% of theoretical after the crude Br2 is dried over sulfuric acid. It just didn't want to happen for me tonight. Much was lost out the top of my
reflux condensor (vent hose is red), and still there is a bunch in solution.
What went wrong:
Added acid just a little too fast (was very hard to see what I was doing due to condensed bromine water plus there is a large hole in my addition
funnel)... that resulted in losing quite a bit out the top of the condensor.
The H2O2 will begin to decompose as soon as the sulfuric is added resulting in loss of oxidant before all the Br2 is released from solution. After I
added the acid, I decided I would try and add some more H2O2 and got another 10 or so mL of product.
Things I would do next time:
Add some salt of some sort to lower the cooling temp down as low as possible.
Change over to simple distillation, put receiving flask under ice use reflux condensor on vacuum takeoff connected by short hose.
Possibly add some H2O2 to the acid to reduce volatility and increase available oxidant.
[Edited on 18-6-2006 by evil_lurker]
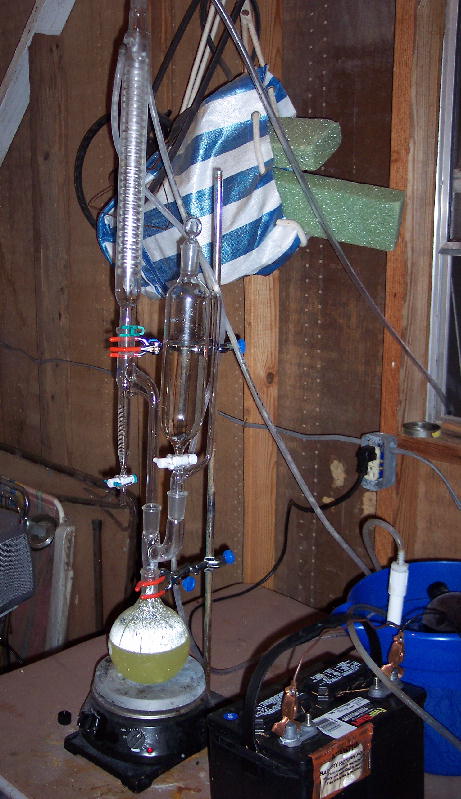
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Bromine in receiver.

|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
Very nice! It's always more fun to make the elements yourself than it is to buy them. The sample of chlorine I have in my element collection is one
of my favorites because I made it myself from calcium hypochlorite and hydrochloric acid. Whenever I get around to it, I hope to make some amorphous
silicon through a reaction between magnesium powder, sand, and HCl.
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Well its nice, but yeilds are still a bit on the low side. I'm hoping for a 60% or better yield.
I'm going to try it again this afternoon. Right now I am adding H2SO4 drop by drop into a saturated solution of NaBr. My reasoning is since the
oxidant for whatever reason gets reduced/lost during the process, it would be better to add it to a solution of HBr than to do it the other way
around. Plus, since the H2O2 is more diluted, it will be easier to contro the rate of addition and give my condensor time to catch up.
[Edited on 18-6-2006 by evil_lurker]
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
MORE LIKE IT!
This time I did a 3 mole batch.
Using the following:
3 moles NaBr were dissolved in just enough hot water to make a saturated solution.
1.5 moles of H2SO4 were slowly dripped in at a sufficiant rate to prevent HBr gas loss.
Heat was added so that the temperature of the HBr solution was over the boiling point of Br2 and left on until the end of the distillation. 12% H2O2
was slowly added at a rate the condensor could keep up with the bromine coming over and was discontinued when it appeard the Br2 began to slack off.
Distilation was allowed to run for a few more minutes then discontinued
Both the cooling water and the reciever flask had CaCl2 added to reduce their temperatures. Very little bromine vapor escaped this way, and in fact I
could see only a trace of Br2 vapor in the top by the vacuum exit.
Yeild has not been measured as of yet, but looks much much better than the last run.
[Edited on 19-6-2006 by evil_lurker]

|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Bromine in bottom of condensor...

|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
And now the results... a bunch of wet bromine!
Total yield is approx 166 grams for this run. Based on available bromine and deducting for water carried over, I'd guess that my percentage is
somewhere in the 60-65% range based on the starting bromine.
Thats still not as quite as high as I'd prefer, but all things considered it would be acceptable to me.
I know its unscientific not to be doing all the measurements, but if I had to guess I'd say there is around 300 grams of Br2 sitting in my deep
freezer.
Which by the way, when I opened the bottle it was under slight pressure even though the bromine had partially solidified!
[Edited on 19-6-2006 by evil_lurker]

|
|
|
Jdurg
Hazard to Others
  
Posts: 220
Registered: 10-6-2006
Location: Connecticut, USA
Member Is Offline
Mood: No Mood
|
|
Wow! That's still quite a bit of bromine there. Nice job! You may want to consider keeping it stored in a dedicated freezer. As a solid, Br2 is
less likely to eat through whatever container you plan on storing it in. If it's kept in an amber glass bottle with a Teflon lid and is tightly
sealed, you can freeze it solid and in the case that there is a power failure the bottle will still keep it relatively safely.
\"A real fart is beefy, has a density greater than or equal to the air surrounding it, consists of the unmistakable scent of broccoli, and usually
requires wiping afterwards.\"
http://maddox.xmission.com. |
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
To celebrate Independance Day I decided to make bromine for the first time. After reading through this thread it seemed wise to just make a small
amount at first. Also I wanted the synthesis to be under good control. Hermann Rymmp's method using MnO2 (provided earlier by jubrail) seemed
perfect.
I added pool spa NaBr and pottery grade MnO2 (which is very fine) to a 250mL RBF then added the dilute H2SO4 (Rooto grade). I could see a haze of
orange gas at first. Using a bunsen burner I began to heat at low heat until drops of dark red liquid slowly began to roll down the condenser. I
only made about 0.5mL but it was still exciting to see it form for first time.
See picture:
[Edited on 5-7-2006 by Magpie]
[Edited on 5-7-2006 by Magpie]
E.b.Chemoleo: reduced resolution of pic to make thread readable.
[Edited on 30-1-2007 by chemoleo]

The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
H2O2 from Sally Beauty Supply is the way to go on the oxidant. Not so cheap, but readily available, and no stinking hazmat fees!
I found a deal on NaBr on ebay, $5.48 per pound with $6.00 flat shipping regardless of quantity. I ordered 6 pounds, and by the time I'm done I hope
to have about 1.5 kilograms of bromine to play with!
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
1.5 kilograms! You (and Bromic Acid) must really love this stuff. How do you store it? I put mine in a small Boston round bottle with Teflon lined
cap. But even that made me uneasy as it is so hot in my lab. So I stuck the bottle in my freezer. It is now nicely frozen. 
For my planned synthesis of bromobenzene I only need about 15 mL. So will have to go back into production on a larger scale.
evil_lurker did you decide that beauty shop H2O2 is OK for this application. Earlier you had some concern about buffers. Beauty shop H2O2 is readily
available in drug stores and would make a convenient oxidant. MnO2 is too messy for large scale work, I think.
My bottle of "Clairol 20" lists water, H2O2, and phosphoric acid as the only ingredients. I wouldn't think phosphoric acid would be a problem here.
[Edited on 6-7-2006 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
evil_lurker
National Hazard
   
Posts: 767
Registered: 12-3-2005
Location: United States of Elbonia
Member Is Offline
Mood: On the wagon again.
|
|
Concerning the buffers, probably not. I'm not for sure, but after I rinsed out the reaction flask and left water standing in it for a coupla days some
unknown "snot" formed inside. That left me to believe that the buffer to be some sort of organic compound which would react with the bromine and
reduce yields. But then I got to thinking about it, all the organics that I know of and hydrogen peroxide don't mix very well. 
I use the 40 volume clear myself. Its like $4.99 for a quart or so and is good for several moles. 20 volume is only double strength OTC hydrogen
peroxide... not much use in my book... simply not concentrated enough.
For storage I been using the amber glass safety coated PTFE lined cap bottles from www.cynmar.com in the freezer. They seem to work well and are NICE. The safety coating makes them a lot more durable... they don't even "clink"
when they come into contact with each other.
As far as why I like bromine so much, there is a lot you can do with it. Substitutes for chlorine very nicely and is much easier to handle.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Everyone is playing with bromine these days it seems!
I did the bromate, bromide, acid method tonight. The ammounts were such that as a maximum I could get 1g of bromine.
Diluted H2SO4 was added to a solution of bromate and bromide while being heated over an alcohol burner. I collected around 0.2 ml or so of bromine,
with double the ammount of water floating above it.
Did I mention I managed to do this indoors with only a window open? Only a
slight bromine smell was noticed near the end, before the system was filled with NaOH solution, and the apparatus disassembled under water, and the
distillation flask containing the most bromine was imersed in the toilet bowl and flushed several times dispersing the bromine solution. Only a
slight bromine smell was noticed near the end, before the system was filled with NaOH solution, and the apparatus disassembled under water, and the
distillation flask containing the most bromine was imersed in the toilet bowl and flushed several times dispersing the bromine solution.
Unless of course it deadened my smell, in which case Ill be dead tomorrow . .
EDIT: I kinda like the smell, seems similar to iodine.
[Edited on 7-7-2006 by rogue chemist]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I did some test tube scale bromine making today:
1. In the 1st I dissolved 0.5g NaBr in 1 mL Clairoxide 20 (6% H2O2) then added about 0.25mL 86% sulfuric acid (Rooto). Bromine production was good.
I added heat to get as much bromine produced as possible.
2. The 2nd test was with 0.25g NaBr and 1 mL 86% sulfuric acid. Bromine production started right away but was weak. Even added heat did not help
much.
Just out of curiosity I calculated the potentials to produce Br2(l) from Br- using various oxidants in acid. Here's the results:
1. MnO2, Br-, and H+................+0.165V
2. MnO4-, Br-, and H+..............+0.445V
3. H2O2, Br-, and H+................+0.705V
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have finished making the bromine (10-15mL) that I need for my synthesis of bromobenzene. I plan to use Br2 and iron filings per my old school
procedure.
The procedure calls for dry glassware. I have noted a bit of ice crystals in my bromine bottle. Vogel says this water can be removed by washing with
con. sulfuric acid. All I have readily available is my Rooto which is 86% vs the 96-98% of con. sulfuric. I hate to find out by "trial & error"
when working with all my bromine. Does anyone know if the 86% would be of sufficient strength?
Duh. Maybe I should just pour the bromine off the ice.
[Edited on 9-7-2006 by Magpie]
[Edited on 9-7-2006 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
| Pages:
1
..
3
4
5
6
7
..
13 |