| Pages:
1
..
3
4
5 |
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
| Quote: | | I really don't want to make a separation using diethyl ether. Perhaps dichloromethane can be used instead? |
I've found that most procedures calling for an extraction with diethyl ether do so to avoid losses from rotovapping down something volatile. I almost
always use DCM myself, due to it being heavier - no reloading the sep. funnel - and the ease of drying it.
Na<sub>2</sub>SO<sub>4</sub> never seems to get diethyl ether dry enough, and MgSO<sub>4</sub> is somewhat soluble
in it.
| Quote: | | Quote: | | I won't be doing the flash chromatography. |
It is not really feasible for something as volatile as cyclohexanol anyway. |
I will second this. I once had no choice but to run a column to isolate something with a b.p. near 100C, and it was a nightmare. I used pentanes/ether
as the eluent, distilled it off carefully, and the yield still sucked. Coincidentally, Aldrich added it to their catalog a few days later, the first
time I ever saw it commercially available.   If the b.p. is much below 200C it's going to be a pain. If the b.p. is much below 200C it's going to be a pain.
| Quote: | | * There are plenty of papers claiming this reagents reduces nitroaromatics to anilines under similar conditions. Besides, my BS detector goes wild in
such cases due to the unfortunate frequency of fictional papers coming from India&Iran&Co. combined with certain weird
things of which maybe the weirdest one is the oxygen content measured with an elemental analysis! Besides, the products are known compounds and they
don't even give melting points and back them up with references. Another improper thing is in that they did not include any references for the
nitroaromatics being reduced with thiourea dioxide. I'm sorry to say this, but whoever was the peer reviewer for this paper truly did a very lousy
job. |
I feel sorry for legitimate scientists from India and Iran trying to establish a career in chemistry as what Nicodem says is the truth. If I'm looking
up a reference and I see "Iran" or "India" I am as inclined to believe their claims as if it were posted here by a member with three posts, two of
which were outright spam. It's especially likely to be bogus if it appears to offer a miraculously simple, obvious solution to a general problem in
synthesis, or is published in Tetrahedron Letters or an obscure journal. (The review process for Tetrahedron Letters is a joke and it sucks. Pharma
sometimes publishes some interesting work there, but it's largely lost in the noise thanks to all of bogus papers. No SI, no procedures, lots of
errors, and outrageous claims - all par for the course.)
[Edited on 28-3-2013 by madscientist]
I weep at the sight of flaming acetic anhydride.
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
This is a continuation of my preparation of cyclohexanol from cyclohexanone by reduction with TUD:
Yesterday I saturated the reaction product with NaCl. The supernate was then decanted to remove the excess solid NaCl. The decantate was then
extracted with 22mL of DCM in a separatory funnel. The DCM separated quickly and cleanly from the aqueous phase. The DCM phase was then washed with
50mL of water in a separatory funnel. The separation here was very slow, but reasonably clean, as can be seen below:

water wash of DCM phase
The organic fraction was then subjected to simple distillation to remove the DCM. This resulted in 4.6g of a yellow tinged liquid that did not
solidify in an ice bath. Freezing point of cyclohexanol is 25.5°C. Today this product was subjected to a short-path distillation as shown below:

short-path distillation
The condensate is 3.6g of clear cyclohexanol as shown below:

distilled cyclohexanol
The boiling point during the distillation was 159.5°C vs a lit value of 161.1°C. The yield is 37.3%. This compares to the yield of 45% found by
Shuan-Liang.
The product did have a camphor-like smell as reported in the literature. It was tainted with some urine smell however as I neglected to remove enough
forerun during the distillation.
I am quite satisfied with these results and wish to thank Nicodem again for his excellent guidance. I feel that if this synthesis was done at a
larger scale, and with scaled back solvent/NaOH/TUD to be more in line with stoichiometry as Nicodem recommended, that %yield would be improved.
edit: I also think that the 4 hr reaction period could be reduced considerably as the insoluble inorganic salts were formed quite early.
Questions, comments, and recommendations are welcomed.
[Edited on 29-3-2013 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
megalodon
Harmless

Posts: 16
Registered: 11-6-2013
Location: behind seven (7) proxies
Member Is Offline
Mood: No Mood
|
|
I'd like to bring up reductive alkylation with TUD again ... has anyone had success with it?
The main hangup I see is in the formation of the imine. The reaction mechanism is catalyzed by protons. On the other hand, these TUD reductions are
done under basic conditions. I wonder if there is a way to reconcile these conditions for a one-pot synthesis?
http://www.masterorganicchemistry.com/2010/05/24/imines-and-...
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I don't think this has been posted yet...
Thiourea dioxide promoted efficient organocatalytic one-pot synthesis of a library of novel heterocyclic compounds
Sanny Verma, Subodh Kumar, Suman L. Jain and Bir Sain
Org. Biomol. Chem., 2011, 9, 6943
| Quote: |
The utility of thiourea dioxide as an efficient organocatalyst
for the library synthesis of novel heterocyclic compounds
via one-pot multicomponent coupling reactions is disclosed.
Thiourea dioxide is an inexpensive and readily accessible
catalyst, resulting in better product yields as compared to the
corresponding thiourea as catalyst. Thiourea dioxide is found
to be insoluble in various organic solvents and therefore at the
end of the reaction products can be separated by extraction
with diethyl ether and the recovered catalyst can be used
several times with consistent catalytic activity.
|
Attachment: 6943-6948.pdf (235kB)
This file has been downloaded 1120 times
Ethyl acetoacetate synthesis: http://www.youtube.com/watch?v=47S3OVwanhY
[Edited on 29-9-2013 by mayko]
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
"A metal free reduction of aryl-N-nitrosamines to corresponding hydrazines using sustainable reductant thiourea dioxide"
| Quote: |
Reduction of aryl-N-nitrosamines to corresponding hydrazines is
reported under metal free condition using sustainable industrial
reductant thiourea dioxide (TDO). The reaction takes place under
mild conditions in aqueous medium to provide good to excellent
yields of desired products. Sensitive functional groups such as
olefin, alkyne and aryl halides were found to be stable during the
reduction while a wide range of aryl N-nitrosamines were
successfully converted to desired hydrazines. One-pot synthesis of
arylhydrazines from corresponding secondary amines via Nnitrosamine
intermediate was established.
|
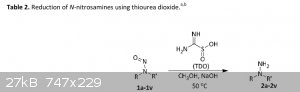
a. Reaction conditions: N-nitrosamine (1 mmol), TDO (3 equiv.), CH3OH (2 mL), NaOH (1 molar, 6 equiv.) were stirred at 50 oC.
http://pubs.rsc.org/en/Content/ArticleLanding/2016/GC/C6GC02...
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Magpie, i have no reason to doubt Shuan-Liang's results, but 45% is a crappy yield for such an easy compound as alkyl substituted
cyclohexanone. Sodium dithionite gives 80% yield of cyclohexanol from cyclohexanone. I would consider thiourea dithionite to be a weaker analog of
dithionite. In fact, aerobic decomposition fo thiourea dioxide leads to dithionite production, while anaerobic decomposition product, sulfoxylate
SO22-, is unstable and a weak reducing agent, decomposing further into bisulfite and sulfide.
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
Yawn, Nitrostyrene with Raney Ni, H2 in MeOH gives a better yield thank you very much. Without the rubbish work up and chasing exotic solvents and
reagents.
TUD might have better luck with other substrates, but the TM in question is very old hat indeed.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Does anybody have an access to document "Reduction of Organic Compounds with Thiourea Dioxide II. The Reduction of Organic Nitrogen Compounds"
translated to english or any document that contains information about reducing aromatic nitro compounds using TUDO?
|
|
|
MJ101
Hazard to Self
 
Posts: 82
Registered: 14-6-2018
Member Is Offline
Mood: Always Sunny
|
|
Quote: Originally posted by UnintentionalChaos  | Hey look, someone (me) actually used TUD for something! I reduced 3-nitrophthalhydrazide to luminol, which is a reduction of an aromatic nitro group.
So, not the most impressive demo of reducing power, but it's something:
http://youtu.be/-PGtoZEZnzc
Reduction at 3:34
Glowing stuff at 19:33 |
@UC: That's why I've watched just about all of your videos. 
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
interesting preprint:
Basoccu, F., Cuccu, F., Caboni, P., Luca, L. de, & Porcheddu, A. (2023). Mechanochemistry frees the thiourea dioxide (TDO) from the ’veils’ of
the solvent, exposing all its reactivity. https://doi.org/10.20944/preprints202302.0062.v1
| Quote: | The synthesis of nitrogen-based heterocycles has always been considered essential in the development of pharmaceuticals in medicine and agriculture.
This explains why various synthetic
approaches have been proposed in recent decades. However, performing as methods often imply harsh conditions or the employment of toxic solvents and
dangerous reagents. Mechanochemistry is undoubtedly one of the most promising technologies currently used for reducing any possible
environmental impact due to the worldwide interest in developing solvent-free synthetic pathways.
Following this line, we propose a renewed mechanochemical protocol for synthesizing various heterocyclic classes by exploiting the reducing
proprieties and the electrophilic nature of thiourea dioxide (TDO). Simultaneously using the ready availability and low cost of a component of the
textile
industry such as TDO and all the advantages brought by a green technique such as mechanochemistry, we plotted the route towards a more sustainable and
eco-friendly methodology for obtaining heterocyclic moieties. |
[Edited on 25-4-2023 by mayko]
Attachment: TUDO_mechanochemistry.pdf (1.1MB)
This file has been downloaded 244 times
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
| Pages:
1
..
3
4
5 |