| Pages:
1
..
46
47
48
49
50
..
77 |
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by fluorescence  | Just discovered while drying my product that Thallium(I)Chromate seems to be thermochromic. Tried it 3 times in a row and it would quickly turn
orange-red while heated even slightly and quickly turn yellow again afterwards. Not sure if this is already in the literature I didn't check yet but
Tl-Chemistry just surprises me everytime I try it.
|
Wow that is really amazing!  I had never chance to work with Tl and its
compounds. I heard about a few mercury compounds that are thermochromic. If you find more about, please share info. Thanks. I had never chance to work with Tl and its
compounds. I heard about a few mercury compounds that are thermochromic. If you find more about, please share info. Thanks.
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
There is an interesting book on this topic. Not for solid state compounds but for coordination compounds called Inorganic Chromotropism. The book
spends severals pages on thermochromism in for example mercury complexes and why and how it changes. Really interesting to read. Unfortuantely I dont
have it I read it once but there is no ebook on it. I might try to get it one day and scan the pages.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Just got a sep funnel! Also, one of those actinic Erlenmeyer flasks and some small volumetric flasks, all of which my AP teacher had decided she
didn't need. *Score*
Edit: At least I think it's a pretty picture...it's the most sophisticated piece of glassware in my lab...Also, it was formerly a
piece used at OSU, our local university...

[Edited on 5-24-2016 by The Volatile Chemist]
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Nice  I got mine in a similar way. Went to a pharmacy when they closed and sold
all their stuff and bought a lot of glassware. I think the seperating funnel was really cheap or even a gift like yours. Cant' really remember I got mine in a similar way. Went to a pharmacy when they closed and sold
all their stuff and bought a lot of glassware. I think the seperating funnel was really cheap or even a gift like yours. Cant' really remember
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
TVC-it IS pretty. Both of mine are only 250ml 
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
mr.crow
National Hazard
   
Posts: 884
Registered: 9-9-2009
Location: Canada
Member Is Offline
Mood: 0xFF
|
|
Quote: Originally posted by The Volatile Chemist  | Just got a sep funnel! Also, one of those actinic Erlenmeyer flasks and some small volumetric flasks, all of which my AP teacher had decided she
didn't need. *Score*
Edit: At least I think it's a pretty picture...it's the most sophisticated piece of glassware in my lab...Also, it was formerly a
piece used at OSU, our local university...
[Edited on 5-24-2016 by The Volatile Chemist] |
That's a nice sep funnel. Its always nice to save old junk from the university.
Double, double toil and trouble; Fire burn, and caldron bubble
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by mr.crow  | Quote: Originally posted by The Volatile Chemist  | Just got a sep funnel! Also, one of those actinic Erlenmeyer flasks and some small volumetric flasks, all of which my AP teacher had decided she
didn't need. *Score*
Edit: At least I think it's a pretty picture...it's the most sophisticated piece of glassware in my lab...Also, it was formerly a
piece used at OSU, our local university...
[Edited on 5-24-2016 by The Volatile Chemist] |
That's a nice sep funnel. Its always nice to save old junk from the university. |
Thanks, indeed!
Arkoma, it's almost too big. But my teacher didn't offer me the 125mL one she had, it was in her Chemis-tree....
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
I'd trade ya in a heartbeat...........................
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Holmium acetate isn't hygroscopic. This happened.
<img src=http://i.imgur.com/IlXJllv.jpg width=800>
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Still discovering new things about the 'Lanths' I see...looks cool! About how much is in the vial, though?
|
|
|
The Plutonium Bunny
Harmless

Posts: 19
Registered: 22-4-2015
Location: On an actinide asteroid
Member Is Offline
Mood: Fissile
|
|
Recently, I've been really interested in crystals of pure metals, mostly for my element collection. After being inspired by The Backyard Scientist's
video (see his post on page 23 of this thread), I decided to grow some of my own copper crystals. Over a period of six weeks, I used a 40g/L solution
of copper sulfate and two copper electrodes with a current of always less than 10mA to grow some truly amazing copper crystal clusters. Unlike nearly
all the other experiments I saw online, these crystals are very large and sharply defined - they are not nodules or rounded growths. I think that the
extremely low current and the very long growth time contributes to the crystalline nature of this growth.
I made a YouTube video about how I grew this, and I also included a 360° rotation to show the entire crystal. https://www.youtube.com/watch?v=zZniOJ7swic
Also, for those who might like to repeat this experiment (the crystal is truly stunning), I wrote an extensive post on my website with details on the
experiment. http://sciencewithscreens.blogspot.com/2016/06/experiment-53-growing-large-copper.html This post also has a link to my experimental data and
observations I recorded during the six-week period.
Finally, I also included the less-impressive picture of my first attempt. This used currents of around 20mA over two weeks, and I accidentally broke
the crystal. It's still pretty neat, though.
In the future, I will be trying other metals. Does anyone have experience with growing crystals of other metals? Thank you!


|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Well, thats... Awesome crystals!
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Bismuth forms nice crystals (see these). You can form them by slow cooling of molten Bi.
[Edited on 6-26-2016 by Metacelsus]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Huh, will have to try the copper crystal thing. I'm bad with the math behind electronics - did you just hook it up to a 9v battery and let it sit, or
did you have to use a resistor? Should probably just check your website...
|
|
|
CrystalCage
Harmless

Posts: 7
Registered: 22-11-2015
Member Is Offline
Mood: No Mood
|
|
First pic: Te metal, Se metal, copper nanoparticles and a copper acetate solution. Te and Se metal was a souvenir during my internship hahahaha  . Copper nanoparticles were synthesized from ascorbic acid and copper acetate
solution. . Copper nanoparticles were synthesized from ascorbic acid and copper acetate
solution.
Second pic: A potassium ferrioxalate crystal. Around 1.5 inches in size.
Grown around 4 weeks, and grows cleanly and fast as well.
Made from dissolving iron with HCl, forming FeCl2 then precipitated into FeC2O4 using oxalic acid, washed away the chlorides with water, then oxidized
into Fe(OH)3 with H2O2 made basic with K2C2O4. The resulting precipitate (Do not decant away the solution!) is then dissolved with oxalic acid again
forming apple green solution.
The brown stain is the reacted solution with light, meaning it is light sensitive.


[Edited on 28-6-2016 by CrystalCage]
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Yes, it's quite light sensitive. Make sure to keep your crystal in the dark whenever you aren't taking it out to show somebody, or else you'll find
after a while that it will start to turn yellow and crumble... I had a few nice ones that this happened to over the course of a year or so, as I had
assumed that they had to be wet to decompose. I was wrong. I have another bag of smaller crystals that were prepared at the same time and kept in a
dark drawer, and they are still brilliantly green.
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by The Plutonium Bunny  | Recently, I've been really interested in crystals of pure metals, mostly for my element collection. After being inspired by The Backyard Scientist's
video (see his post on page 23 of this thread), I decided to grow some of my own copper crystals. Over a period of six weeks, I used a 40g/L solution
of copper sulfate and two copper electrodes with a current of always less than 10mA to grow some truly amazing copper crystal clusters. Unlike nearly
all the other experiments I saw online, these crystals are very large and sharply defined - they are not nodules or rounded growths. I think that the
extremely low current and the very long growth time contributes to the crystalline nature of this growth.
|
Hi man,
very nice work! I decided to set up the same experiment and grow my own crystals. I used LM317 as well with 2x120 ohm resistor and potentiometer on
adjustement pin of LM317. This resulted in 0.30V between cathode and anode but I could not measure current flowing through the circuit. Display stayed
at 0.00 mA. Something must have gone wrong. Would you mind posting your LM317 circuit scheme and how did you measure volatge and current?
Thanks a lot! 
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
The Plutonium Bunny
Harmless

Posts: 19
Registered: 22-4-2015
Location: On an actinide asteroid
Member Is Offline
Mood: Fissile
|
|
Quote: Originally posted by Hegi  |
Hi man,
very nice work! I decided to set up the same experiment and grow my own crystals. I used LM317 as well with 2x120 ohm resistor and potentiometer on
adjustement pin of LM317. This resulted in 0.30V between cathode and anode but I could not measure current flowing through the circuit. Display stayed
at 0.00 mA. Something must have gone wrong. Would you mind posting your LM317 circuit scheme and how did you measure volatge and current?
Thanks a lot!  |
Certainly! Here is a schematic I drew in Paint of my setup. The five 1N4007 diodes drop the output voltage a little bit so that I can get lower
output voltages with the LM317. Also, the resistors and potentiometers don't have to be exact- adjustments will still give you a wide range of
voltages.
Also, something I learned a while back is that to measure current with a multimeter, the electricity must flow through the multimeter. This is
different from measuring voltage, where the multimeter connects to two opposite points. Measuring current requires breaking the circuit and
connecting the multimeter to either end of the break. It took me a while to learn this. 
Good luck, and keep trying! It will be worth it in the end!
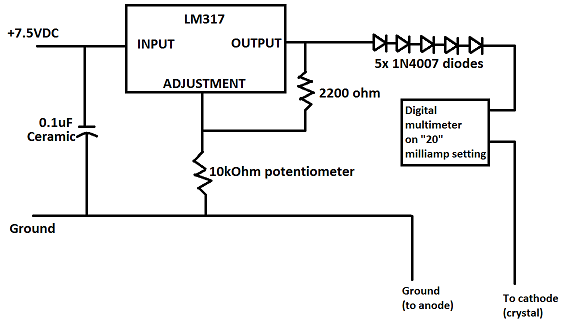
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It's always a good idea to decouple the ADJ input and have at least a bit of smoothing on the output, thusly :
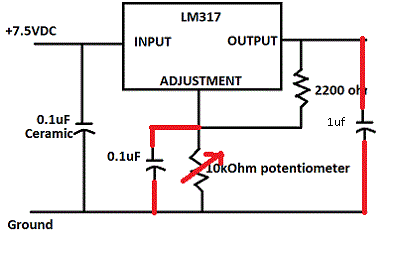
If not, there is a risk of oscillation which is hard to see on a digital multimeter.
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Thanks guys, my scheme is the same (except i did not use capacitors)... After 24 hours dendritic copper crystals about 0.3-0.4 mm size can be
observed. I did measurement of current in the way it is supossed to be measured but NOTHING happened - value stays at 0.00 mA. Voltage is in range
0,27-0,30V and the resistance of the solution is approximately 220 ohm.
Did you observe forming small dendrites at the begining? .. or should I start again? 
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I think hackaday did a little article on this prep, was that the result of anyone here? I think it had the first image posted in the article.
|
|
|
alexleyenda
Hazard to Others
  
Posts: 277
Registered: 17-12-2013
Location: Québec, Canada
Member Is Offline
Mood: Busy studying chemistry at the University
|
|
Woah, the copper crystals are amazing, I must try that one day.
A colorful reaction to make a new compound and its crystals during my internship.
[Edited on 9-7-2016 by alexleyenda]
 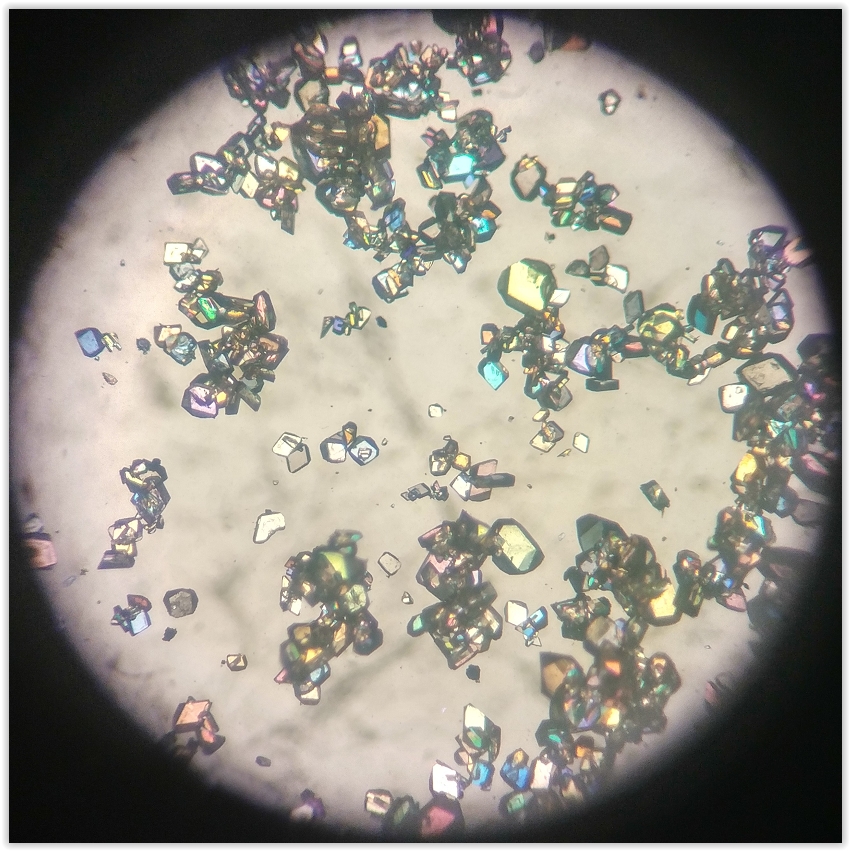
Help us build the Sciencemadness Wiki! Every question and tips about amateur chemistry two clicks away, wouldn't that be awesome?!
sciencemadness.org/smwiki
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
The effect of 8bar water on the seat of my sink water tap.
Its soft water but it may have been caused by inclusions in the poor quality brass or cracks.

|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by wg48  | The effect of 8bar water on the seat of my sink water tap.
Its soft water but it may have been caused by inclusions in the poor quality brass or cracks.
|
Soft water is better/gentle for resistance into warming systems but it is also more corrosive to plumbing and piping than hard water.
No pain, no gain   ...no universal curing agent ...no universal curing agent 
[Edited on 14-7-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
This compound I made (at the research lab where I'm working this summer) shows drastically different fluorescence in solution (30 mg/mL in
CDCl3) vs. as a solid.

[Edited on 7-20-2016 by Metacelsus]
|
|
|
| Pages:
1
..
46
47
48
49
50
..
77 |