| Pages:
1
..
45
46
47
48
49
..
77 |
Firmware21
Harmless

Posts: 33
Registered: 22-1-2015
Member Is Offline
Mood: Ņ͈̣̭̺̈ͬ͊̔i̓̿͑ͯ̂ͪ҉̸̺̀t͉̣͕͙̟̪̅͐͂̏͌ͭ͗̑͝ṙ̶̛̙̥̝̻̟̓ͬ̾ͧ͒͘ͅa͒͊ͯ̾̑̏̌̓̕҉͚͚͓͔͙͚̥t͆̌͑ͩ̐͗
͖̻̲̪̲̙͘ͅe͙͕͙̙̤̤̫̒ͩͣ̅̊̍̉͒ͬ́͟ͅs͈̬̮̥̻͂ͥͨ̂ͮͨ͒́͠ !!!
|
|
This is why I love copper salts: Easy to make and beautiful.
It's Copper II chloride in excess HCl

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Wow looks really cool, I like green compounds ^^.
Did you even put some hazard labels on the bottle ?
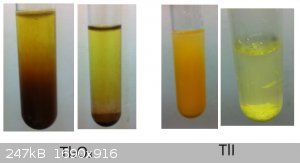
So here are two other Thallium compounds. As promised I made some Tl(III) Oxide from Ferricyanide(III), KOH and Tl-Nitrate.
And on the right is some Thallium(I)-Iodide. I tried some Tl(III)Iodide as well but it wouldt form. I love how the color changes from the fine
powdered orange iodide to the bigger yellow chunks after standing for 5 min. or so.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Nice barium compound 'Deathhunter'! Very reminiscent of strontium chloride, which I'd recently done the same with.
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Patterns in a flame reminiscent of the patterns on the cathode of a low pressure discharge tube.


|
|
|
Firmware21
Harmless

Posts: 33
Registered: 22-1-2015
Member Is Offline
Mood: Ņ͈̣̭̺̈ͬ͊̔i̓̿͑ͯ̂ͪ҉̸̺̀t͉̣͕͙̟̪̅͐͂̏͌ͭ͗̑͝ṙ̶̛̙̥̝̻̟̓ͬ̾ͧ͒͘ͅa͒͊ͯ̾̑̏̌̓̕҉͚͚͓͔͙͚̥t͆̌͑ͩ̐͗
͖̻̲̪̲̙͘ͅe͙͕͙̙̤̤̫̒ͩͣ̅̊̍̉͒ͬ́͟ͅs͈̬̮̥̻͂ͥͨ̂ͮͨ͒́͠ !!!
|
|
@Fluorescence , Yes, I made them with a label printer. The glue on it is too weak to hold the hazard signs in place, so I had to put some tape...
Here's my whole "collection" of componds (including a some elements).
A lot is "homemade".

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Nice,
I like these bottles. Just bought 10 of them last week. But they are crazy expensive here.
So I need to clean them if I dont need them anymore. But yeah really quite useufll.
I see you wrapped some tape around them as well. Although we sometimes stored solvents in them
at the university they seem to let some water through so for many anhydrous compounds I made
without tape around them they werent tight enough.
|
|
|
rasiel
Harmless

Posts: 20
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
Chlorine
Another poster requested I upload a photo of my sample of pressurized chlorine. Just a little ampule cast inside a much larger cube of high quality
resin.

I'm setting a goal of photographing each of the elements in case anyone has any requests :-)
Rasiel
|
|
|
rasiel
Harmless

Posts: 20
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
Wooops, I meant for this to show up on the pretty pictures thread. What'i do wrong?? 
|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
That's beautiful and damn amazing dude
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
What resin did you use? I'm all ways looking for good clear resins like that.
|
|
|
Texium
|
Threads Merged
6-5-2016 at 06:43 |
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
No problem, merged now.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Looks really well done. Some guys I knew tried that as well but after some time they tend to get all brown and cloudy. I hope your resin will stay as
it is at the moment. Looks really nice.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fluorescence  | | Looks really well done. Some guys I knew tried that as well but after some time they tend to get all brown and cloudy. I hope your resin will stay as
it is at the moment. Looks really nice. |
Sounds like they bought cheap resin without UV protectants.
|
|
|
rasiel
Harmless

Posts: 20
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
PMMA, commercial acrylic... it's the stuff Plexiglass (aka Lucite) is made of. The problem with this resin is that it only cures under heat *and*
pressure. If you have an autoclave then give it a shot. It's by far a more presentable option than epoxy which hardens yellow from day one or
polyester which is a pain to work with and shows casting seams even when perfectly poured.
However, the resin is not the only consideration. To get those perfectly polished and straight walls with razor sharp edges can only be done by
machine. With a lathe or mill you might be able to get a really pro-looking finish but I don't personally have experience in this area.
Anyway, I don't know of a US-based supplier for PMMA but there are a number of places that will sell it to you from China on Alibaba. This is what
you're looking for http://goo.gl/L0ycb1
Rasiel
|
|
|
rasiel
Harmless

Posts: 20
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
For those interested in seeing how I'm making the cubes here's a brief but very interesting video that runs through the steps (and you'll see why it's
not something easily replicated at home)
https://www.youtube.com/watch?v=hNaOKrE643g
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Beatiful green-blue crystals of a-modification of NiSO4.6H2O.
Im still growing them, the biggest is about 1cm in diameter  . .
EDIT: I didnt notice that hegi has already posted this picture in another thread, should I delete this post?

[Edited on 11-5-2016 by crystal grower]
|
|
|
rasiel
Harmless

Posts: 20
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
Oh wow, they look like breath mints!
:-)
Rasiel
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Lol, they remind me sapphires  . .
|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by rasiel  | Another poster requested I upload a photo of my sample of pressurized chlorine. Just a little ampule cast inside a much larger cube of high quality
resin.
I'm setting a goal of photographing each of the elements in case anyone has any requests :-)
Rasiel |
A potential problem with potting glass in resin is if the resin contracts as it hardens it can crack the glass. This can happen years after the item
is potted.
My mother had a hour glass egg timer given to her. It was was potted in a clear block of resin. About a year after she received it it stopped working.
The sand would no flow thru the constriction. On close examination there was a crack in the glass thru the constriction. The egg timer was kept as an
ornament. Over a few more years many more cracks appeared in the glass and distortion at the surface of the block over the ends of the hour glass
could be seen.
I worked on a high voltage power supply that had been potted in a rigid resin. The high failure rate in the field was eliminated when it was potted
in silicon rubber.
Of cause in the above cases the wrong resin or incorrect mixing or cutting temperature could have been the cause.
I guess a test block could be made by potting a glass rod in one side of long block to see if it bends.
|
|
|
rasiel
Harmless

Posts: 20
Registered: 8-2-2016
Member Is Offline
Mood: No Mood
|
|
Today I took a pic of calcium... the way few people get to see it!

|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Some Se82+ cations forming. The first run was too concentrated and appeared almost black this one here is really dilute and
still it has a quite strong green color.
For those who actually read my bio ^^, I do a lot of Selenium Chemisry for my Thesis. Certainly not an element I would work with if nobody had asked
me to do it. I love toxic compounds but Selenium is just really nasty. And most of my Selenides at work fall apart on air producing some
H2Se and it's really terrible to work with that.
I think that Selenium chemistry is something most people have no clues about. If wanted I could do a small article on the forum on actual selenium
chemistry and behaviour in comparison to sulfur for example.
Also has anyone made some NaHSe before. I tried yesterday via the Se + NaBH4 in Ethanol method and it didn't really work.

|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I'd love to hear more about selenium chemistry. It will save me actually doing it. 
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Yeah avoid ! Such a nasty stuff. I was preparing a sample for the powder diffraction and only used the tip of the of the spatula like the tiny edge
and had that on the sample carrier which was no pure Selenide but a mixture of my products and some Sesquiselenide that had formed. I couldt wash it
since due to the Selenide it formed a lot of red selenium powder all over the sample as soon as it touched water and so I washed it together with the
flux which was RbCl I think ? So the RbCl was really dry and started to suck up water from the air turning my sample into an H2Se Generator xD. The
small amout of some tiny grains of Selenide exposed to air was enough to fully cover the complete hallway between my office and the diffractometer
room that even as all doors were opened half an hour later when I came back you couldt walk there !!! That stuff is incredibly nasty. And I spent the
whole day with the complete sample under the microcope. At one point it just cant get any worse. I always wanted to make H2Te but I think there is no
amount tiny enough to actually do it. And that smell is so damn characteristic. H2S doesnt smell like garlic as people say it smells like rotten eggs
H2Se smells a lot like garlic it isnt even that unpleasant because I think I know this smell from somewhere but it's usually too concentrated to be in
a room with it. Like with chlorine gas, a tiny amount is just enough for your nose.
And also what I found really interesting is that as some might know elemental Selenium actually smells. I always thought this was H2Se and it probably
is H2Se but the smell coming from a bottle of Se is a bit different to the smell of real H2Se. Not sure if this is due to concentration but those two
have some differences.
Maybe one day I'll do some tellurium chemistry to try this out as well. Who knows.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Just discovered while drying my product that Thallium(I)Chromate seems to be thermochromic. Tried it 3 times in a row and it would quickly turn
orange-red while heated even slightly and quickly turn yellow again afterwards. Not sure if this is already in the literature I didn't check yet but
Tl-Chemistry just surprises me everytime I try it.

|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Wow o.O, that's indeed interesting.
|
|
|
| Pages:
1
..
45
46
47
48
49
..
77 |