| Pages:
1
..
45
46
47
48
49
..
104 |
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
If you take a nitro group and react with with a grignard of the XMgNHR type you get the below
R-NO2 + XMgNHR' --> R-N(O)=N-R' very useful for energetics synthesis...
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
won't the H of the XMgNHR destroy the grignard reagent formed since its acidic ? I think you meant XMgN(R)2.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by gluon47  | | Can nitric acid be feasibly produced via the reaction of potassium nitrate and hydrochloric acid? I've seen many vids of people making it with
sulphuric acid and potassium nitrate but none using hydrochloric acid. |
In principle it seems a good idea because HCl is a much stronger acid than HNO3...
But practically it is not a good idea because:
-HCl is much more volatile than HNO3
-HCl reacts with HNO3 to form nitrosyl chloride O=N-Cl (see aqua regia) and thus the mixes of HCl/HNO3, NaCl/HNO3 or NaNO3/HCl will be able to
dissolve nearly all metals ... even nobles ones (gold, platine, rhodium,...)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by CuReUS  |
won't the H of the XMgNHR destroy the grignard reagent formed since its acidic ? I think you meant XMgN(R)2. |
@CuReUS,
Why would XMgNH-R be acidic?
If R is alkylic, I suspect it wouldn't be (well everything has something more acid or basic than itself that would make it look like an acid or a base
but that's another debate --> solvent effect).
Without knowing what R is hard to tell!
Also if it was acidic would'nt it form (XMg)2N-R?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by The_Davster  | If you take a nitro group and react with with a grignard of the XMgNHR type you get the below
R-NO2 + XMgNHR' --> R-N(O)=N-R' very useful for energetics synthesis... |
@The_Davster,
Do you have a reference, link or document for me?
That reaction is very interesting for the energetic materials field ... and I'm passionnate about those...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Wolff-Kishner reduction.
I found quite an interesting process on the Internet called the Wolff-Kishner Reduction,which goes as follows:
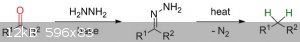
How are the two nitrogen atoms in the intermediate removed to give the final methylene group?
Does it mean that propane/butane can be synthesized from Acetone and MEK respectively?
[Edited on 21-12-2015 by CitricAcid]
|
|
|
User123
Harmless

Posts: 40
Registered: 31-10-2015
Member Is Offline
Mood: No Mood
|
|
The scheme is incomplete. It uses a strong base such as sodium hydroxide to abstract the hydrogens on the outer nitrogen atom and form first a double,
then a triple bond between the two nitrogens. When the triple bond forms the nitrogen-carbon bond breaks and (I don't know whether 'formally' or
in-reality) a carbanion is formed which abstracts a proton from the solvent (I think that ethylene glycol is used, but I'm not sure).
Yes, you can make propane and butane. It is a robust reaction, with good yields, but it can only be used on substances that can withstand the harsh
conditions, and is not a very good general reaction.
[Edited on 21-12-2015 by User123]
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by User123  | The scheme is incomplete. It uses a strong base such as sodium hydroxide to abstract the hydrogens on the outer nitrogen atom and form first a double,
then a triple bond between the two nitrogens. When the triple bond forms the nitrogen-carbon bond breaks and (I don't know whether 'formally' or
in-reality) a carbanion is formed which abstracts a proton from the solvent (I think that ethylene glycol is used, but I'm not sure).
Yes, you can make propane and butane. It is a robust reaction, with good yields, but it can only be used on substances that can withstand the harsh
conditions, and is not a very good general reaction.
[Edited on 21-12-2015 by User123] |
Do you mind giving a theoretical setup to make your explanation more understandable in practice? Suppose that Acetone is used to make propane,how
will the entire setup work/look like?
[Edited on 21-12-2015 by CitricAcid]
|
|
|
User123
Harmless

Posts: 40
Registered: 31-10-2015
Member Is Offline
Mood: No Mood
|
|
You'd just have a flask with acetone hydrazone dissolved in ethylene glycol. To that you would add NaOH or KOH, heat it to 180C or so, and propane
would come flying out of the top!
Is that what you mean?
If you had a non-trivial substrate -- say the product was n-hexylbenzene -- then it would be dissolved in the ethylene glycol, and you would let it
cool, add water, then extract the n-hexylbenzene with a solvent.
[Edited on 21-12-2015 by User123]
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Hydrazine
Is there any way to isolate hydrazine from mixing bleach and household ammonia without adding sulfuric acid? What I mean is:Is there any way to
separate hydrazine from everything else that is formed when mixing bleach and ammonia? Can it be separated via sep funnel?
[Edited on 22-12-2015 by CitricAcid]
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Iodine
Is there any source that sells iodine crystals to individuals?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2733
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: No Mood
|
|
Ebay has a few sources for 50g/$10.
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Since that was a very quick thread,I'll ask another question: Is there any good source of phosphorus? I intend to use it to make PBr3 for use in
brominating alcohols.
|
|
|
Texium
|
Threads Merged
21-12-2015 at 19:49 |
Agari
Banned
Posts: 160
Registered: 8-10-2015
Location: The Amine Group
Member Is Offline
Mood: Lowest Oxidation State
|
|
Quote: Originally posted by CitricAcid  | | Since that was a very quick thread,I'll ask another question: Is there any good source of phosphorus? I intend to use it to make PBr3 for use in
brominating alcohols. |
Onyxmet.com sells phosphorus, J_Sum1 claims to have bought from them more or less frequently,you can ask him if you have any questions about it.
Edit:I doubt you can cleanly separate hydrazine from solution,the densities of household bleach,ammonia,and hydrazine are very similar,and it is also
miscible in water,so you will likely need to chemically separate them.
[Edited on 22-12-2015 by Agari]
Element Collection Status:
Elements Acquired: 21/91
Latest: Lead (Pb)
Quantity: 12g
-----------------------------------------------------
|
|
|
CitricAcid
Harmless

Posts: 28
Registered: 13-12-2015
Member Is Offline
Mood: No Mood
|
|
Back to my original hydrazine question: In addition to isolating it without converting it to its salt form,is there any combination of solvents that
can be used to extract it from solution?
|
|
|
arkoma
Redneck Overlord
      
Posts: 1761
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Benzyl alcohol, Cyclohexanol--KCr2O7 and KMnO4 kin possession. Expected products from oxidations?
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
My first year chemistry tells me alcohols get oxidised first to aldehydes then carboxylic acids. Because these are such strong oxidisers however you
almost certainly won't get any aldehydes, and because this is the real world you might well also get black goo as the primary product.
pH will also play a role in the oxidations, but that's above my skill level.
I suggest smelling the reaction mixture in the benzyl alcohol experiment. Strong smell of vomit = you have produces benzoic acid.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Citric acid, I think you may be confused.
You don't need red P and iodine plus the Wolff-Kishner to reduce epinephrine, one is enough!
If your meth lab can't make hydrazine, you can use the Clemmensen reduction. All you need is Zn/Hg, HCl and ETOH.
But first you must oxidize epinephrine to its ketone.
If you're still having trouble I've got a friend at the DEA who will be glad to 'wrap things up'!
Or do you really want to waste acetone and hydrazine to make propane? Really?
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Benzyl alcohol would be oxidized as Oscilllator describes, first to benzaldehyde and then to benzoic acid. Though I'm pretty sure benzoic acid doesn't
have much of an odor, so I don't know where the vomit thing is coming from.
Cyclohexanol, however, is a secondary alcohol, so it will be oxidized to a ketone- cyclohexanone- and no further.
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by zts16  | Benzyl alcohol would be oxidized as Oscilllator describes, first to benzaldehyde and then to benzoic acid. Though I'm pretty sure benzoic acid doesn't
have much of an odor, so I don't know where the vomit thing is coming from.
Cyclohexanol, however, is a secondary alcohol, so it will be oxidized to a ketone- cyclohexanone- and no further. |
woopsie, I thought he meant butanoic acid. See, this is what happens when you don't do chemistry for a while - stay away from not doing chemistry
kids!
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Online
Mood: Semi-victorious.
|
|
If you oxidize cyclohexanol with permanganate, you will probably get adipic acid.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Online
Mood: PhD candidate!
|
|
Oh sure enough, I forgot
about that possibility. Jones oxidation with dichromate should still yield cyclohexanone though.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I'll add a little to the correct answers above.
1)
Either oxidizer will turn benzyl alcohol to benzoic acid (with good yields). You can stop the oxidation at benzaldhyde by excluding water (use
pyridinium chlorochromate)
2)
Cr(VI) will oxidize cyclohexanol to cyclohexanone. Mn(VII) will do that too... then it will likely break the carbonyl carbon-alpha carbon bond,
eventually yielding hexanedioic acid! (Well really K/Na hexanediate as the reaction requires a base.)
|
|
|
Deathunter88
National Hazard
   
Posts: 519
Registered: 20-2-2015
Location: Beijing, China
Member Is Offline
Mood: No Mood
|
|
Are there any soluble barium compounds that can be used to test for the sulphate ion other than barium nitrate and barium chloride?
I ask because I just recently found out that barium chloride is listed as an "extremely toxic" chemical in China and thus illegal for an individual to
possess. Barium nitrate on the other hand is listed as an explosives precursor and also illegal for the individual to own.
OR if no such compound of barium exists then suggestions of any other compounds that can be used to identify the existence of sulphate ion in solution
would be welcomed.
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Deathunter88  | Are there any soluble barium compounds that can be used to test for the sulphate ion other than barium nitrate and barium chloride?
I ask because I just recently found out that barium chloride is listed as an "extremely toxic" chemical in China and thus illegal for an individual to
possess. Barium nitrate on the other hand is listed as an explosives precursor and also illegal for the individual to own.
OR if no such compound of barium exists then suggestions of any other compounds that can be used to identify the existence of sulphate ion in solution
would be welcomed. |
The barium salts are considered extremely toxic because of the barium. Changing the anion won't change anything, though it may do so regulation-wise.
Is BaCO3 similarly regulated? You can just dissolve it in HCl and filter any unreactive goop off to make a BaCl2 solution.
Strontium salts also produce a precipitate with sulfate and are far less toxic, but are considerably less sensitive.
[Edited on 23-12-2015 by UC235]
|
|
|
| Pages:
1
..
45
46
47
48
49
..
104 |