| Pages:
1
..
43
44
45
46
47
..
77 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I also have a good stones, gemstones and fossiles collection 
Also some other not yet exposed in my glass shelf...





PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
And a few close up of my bismuth cristals:





PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Wow that's gorgeous.
Do you buy minerals or you dig the them yourself ?
And also what purity bismuth do you use?
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Amazing, all of these.
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
I started my collection at the age of 8 (I'm 41 by now), but my personal findings are a minority (less than 0.5% of my collection); most of my
collection come from mineral and fossiles fairs...the other part from tiny gifts.
I focus on beautiful gemstones, usually transparent and colored ranging from half-precious to precious, but at a good price and in natural state or
just pollished.
The last 5 years I have bought some facetted gemstones, some artifical, thus for a very good price...some are huge.
For the last decade I also bought minerals for the chemical content (Beryls for Berylium, some rare minerals for Ytrium and Selenium, Cinnabar for
Mercury, crystaline Silicium, crystaline Bismuth,Stilbine for Antimony, ...)
The Bismuth crystals are probably very pure, I guess they come from the industrial refining process of Copper, Lead and Tin (from cooling
chemineys)...they are colourfull and very advertising if you look at them with a magnifier or microscope...you feel like flying into old Maya
temples...
[Edited on 3-3-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
woelen
Super Administrator
        
Posts: 8012
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I made an ampoule with IBr. Very weird crystals are formed in the inside of the ampoule:
This is the ampoule:

You can see some solid IBr at the bottom of the ampoule, the brown/pink vapor above it, and beautiful crystals at the bottom half of the ampoule.
Here follow some closeups of the crystals:
Crystals on the far side of the ampoule:

Crystals on the near side of the ampoule:

Crystals all around the ampoule:

One week later, the crystals disappeared at one side of the ampoule, but they are still present on one side:

|
|
|
wg48
National Hazard
   
Posts: 821
Registered: 21-11-2015
Member Is Offline
Mood: No Mood
|
|
Fascinating pics woelen. The form of the crystals on the glass sides is reminiscent of the frost patterns on glass. I imagine molecules of the IBr
bouncing a round in the tube and only sticking to the crystals with some probability depending which facet of the crystal they hit.
|
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
Quote: Originally posted by woelen  | I made an ampoule with IBr. Very weird crystals are formed in the inside of the ampoule:
This is the ampoule:
You can see some solid IBr at the bottom of the ampoule, the brown/pink vapor above it, and beautiful crystals at the bottom half of the ampoule.
Here follow some closeups of the crystals:
Crystals on the far side of the ampoule:
Crystals on the near side of the ampoule:
Crystals all around the ampoule:
One week later, the crystals disappeared at one side of the ampoule, but they are still present on one side:
|
Wow, that's really weirdo  . .
What melting point does it have?
I want to try growing some iodine crystals, too bad that I can't find any iodine for a reasonable price (local eshops offers only big quantities so I
can't afford it) and I won't probably get enough from povidone  . .
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
If you store bromine in the freezer, it too forms these beautiful feathery crystals on the walls as it sublimates. Mine is stored in the door, and the
temperature gradient is sufficient that all of the material migrates to the one side of the upright bottle.
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Quote: Originally posted by crystal grower  | Quote: Originally posted by woelen  | I made an ampoule with IBr. Very weird crystals are formed in the inside of the ampoule:
This is the ampoule:
You can see some solid IBr at the bottom of the ampoule, the brown/pink vapor above it, and beautiful crystals at the bottom half of the ampoule.
Here follow some closeups of the crystals:
Crystals on the far side of the ampoule:
Crystals on the near side of the ampoule:
Crystals all around the ampoule:
One week later, the crystals disappeared at one side of the ampoule, but they are still present on one side:
|
Wow, that's really weirdo  . .
What melting point does it have?
I want to try growing some iodine crystals, too bad that I can't find any iodine for a reasonable price (local eshops offers only big quantities so I
can't afford it) and I won't probably get enough from povidone  . .
|
http://www.ebay.com/itm/25-grams-0-88oz-pure-elemental-iodin...
Pretty high purity; this is the time in which products from China should be considered. I ordered a smaller amount before, which came out to be
legitimate, but I should note that they do tend to lie to the customs(as in my case, they labeled the iodine as "clothing accessories" ).
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
crystal grower
Hazard to Others
  
Posts: 474
Registered: 3-1-2016
Location: Os Petrosum
Member Is Offline
Mood: Puzzled
|
|
That seems like a good deal but can I trust ebay? (I haven't bought anything from it yet). 5$ isn't very much so I could try it.
Sorry for being off-topic.
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I have bought Chinese iodine from eBay a couple of times. Last time was 100g a couple of months ago. Good experiences both times.
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
J_sum1, did you have to contact customs and have it cleared first? I've wanted to buy some iodine/potassium iodide off eBay but wasn't sure if I could
legally get it through customs
[Edited on 6-3-2016 by NedsHead]
|
|
|
j_sum1
Administrator
       
Posts: 6320
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Nope. I just ordered it and it arrived. I will U2U my eBay seller but it is undoubtedly not the only possible source.
Like anything, you might expect a little visit from our friends in blue. (And I don't mean the smurfs.) Be ready for that. I have had one visit and
it was painless enough. They said they would record me as legit and I would be unlikely to get another visit for a while.
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Well....that didn't really work out as expected. So this was supposed to be Hexamminecobalt(III)oxohexacarbonatotetraberyllate but I just recently
found the description of how to actually prepare it and I should have had used a Carbonate...so from the fact that even the so called insoliuble
Co(III)ammine-Complex didnt really precipitate and that stuff is still full of Ammonia its probably just some Be(OH)2 that has formed.
I just read that even that complex mentioned above is supposed to be white when clean...so no colored Be compound again which is really sad. I'd
really like to show you that Be Chemistry can be interesting, too but there is just no good inorganic complex which would be colored. s-Orbitals don't
split so there is no transition unfortunately.
I think the only way to make it a bit more colorful would be to bind it to a pi-System that is already colored like in Chlorophyll for Magnesium.
So I will try to prepare the complex mentioned above again in a pure form and hopefully give you a real picture of it. Does anyone know about a good
inorganic perhaps colored Be Compound ?

|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
A slight break from the preceding, but...
I hope nobody objects to explicit images of naked flames?

Candle in microgravity

Direct photographs of sooting n-C4H10 non-premixed gas-jet flames at 1 µg at Reš42, jet diameter 10 mm, showing evidence of
thermophoresis-induced agglomeration at µg.

"CH4/Air premixed flame attached to a carbon-coated brass matrix cooled with water. Fuel rich to fuel lean from left to right and top to
bottom by increasing air flow rate and decreasing CH4 flow rate. Small flames dance around and a butterfly appears. When the butterfly
flies away, flame is gone."
Last not least:
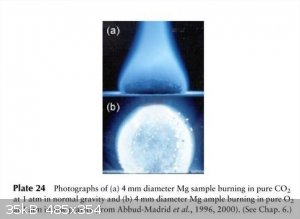
Why you don't extinguish Mg fires with a carbon dioxide extinguisher - even on a space station
|
|
|
Velzee
Hazard to Others
  
Posts: 381
Registered: 19-8-2015
Location: New York
Member Is Offline
Mood: Taking it easy
|
|
Wow!
Check out the ScienceMadness Wiki: http://www.sciencemadness.org/smwiki/index.php/Main_Page
"All truth passes through three stages. First, it is ridiculed. Second, it is violently opposed. Third, it is accepted as being self-evident."
—Arthur Schopenhauer
"¡Vivá Cristo Rey!"
—Saint José Sánchez del Río |
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Ive brought this up before, but since the last pretty pictures thread topped at 40 pages, and was deemed hard to manage with forum software, shouldn't
the same be done here? Or is the software better now?
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
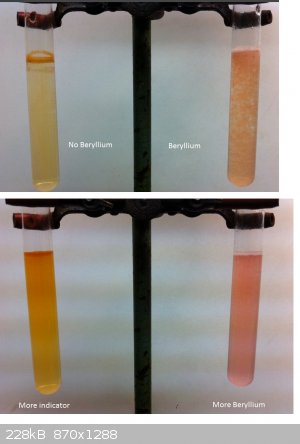
So did another attempt to make a colored Beryllium Compound. This time I used an organic ligand. It took me quite a while and a few tries to get a
useful result but it looks quite cool right now.
According to literature there is a Curcumin complex with Beryllium in an alkali solution. Literature suggests using Ammonia but I found that the
results are so dark if you use too much indicator that you wont be able to see anything. So I switched to KOH as base.
What you see here are two test tubes filled with the same amount of KOH solution, made from one spatula of KOH in about 30 ml of water.
To one I added a Berylliumsulfate soltuion, made from 2 small spatulas of pure BeSO4 in about 25 ml of water. I took about 3-4 ml of this solution and
added it to one of the test tubes and to the other one the same amount of water.
Literature often talks about the precipiate formed (BeOH2) but I did a run with quite much precipitate and it didn't really change that
much. The one you see here is only a little cloudy and its really hard to even see the Hydroxide formed. So this can be done in a quite low
concentration as well. I then added a few drops of curcumine in Ethanol to both of them.
The one without Beryllium stayed in solution and became brown-orane the other one precipitated and changed to a red color (hard to see on the photo).
In the pictures on the bottom I added a lot of indicator to the left test tube without beryllium and some solid BeSO4 to the other one as you can see
as long as they are diluted even higher conc. of indicator and Be althought the both form a red layer where they meet differ in color if you diluted
them long enough.
So I know Quinalizarin looks better but that is too expensive for me to buy it so I stay with curcumin but will try something interesting, soo.
|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Copper(II) acetylacetone
Copper(II) acetylacetonate recrystallized from chloroform... Check out article about preparation - COPPER ACETYLACETONATE PREPARATION
 
[Edited on 23-3-2016 by Hegi]
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
Those look like amazing crystals! I'm interested in viewing the prep but it seems your link doesn't work?
Sadly my acetylacetone synthesis failed, maybe I'll try again in a few weeks...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4332
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Zephyr  | Those look like amazing crystals! I'm interested in viewing the prep but it seems your link doesn't work?
Sadly my acetylacetone synthesis failed, maybe I'll try again in a few weeks... |
Try this: http://chem.pieceofscience.com/?p=990
Hmmm....there's also this: http://www.magritek.com/wp-content/uploads/2015/03/Lab-Manua...
I wonder if I could make some acac. Or if dimethyl malonate would form similar complexes.
[Edited on 23-3-2016 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
Wonderful, thanks DraconicAcid. I look forward to more great projects from pieceofscience!
Here's a pic of the drying rack I built for some substance:

|
|
|
Hegi
Hazard to Others
  
Posts: 199
Registered: 27-9-2013
Member Is Offline
Mood: No idea.
|
|
Quote: Originally posted by Zephyr  | Those look like amazing crystals! I'm interested in viewing the prep but it seems your link doesn't work?
Sadly my acetylacetone synthesis failed, maybe I'll try again in a few weeks... |
Thanks Zephyr! It should work now. What reaction did you try for acetylacetone synthesis?
Our webpage has been shut down forever cause nobody was willing to contribute. Shame on you all!!!
|
|
|
Zephyr
Hazard to Others
  
Posts: 341
Registered: 30-8-2013
Location: Seattle, WA
Member Is Offline
|
|
Here is the synthesis I attempted. As you can see moisture leads to issues, and I suspect it was the culprit... A bit of
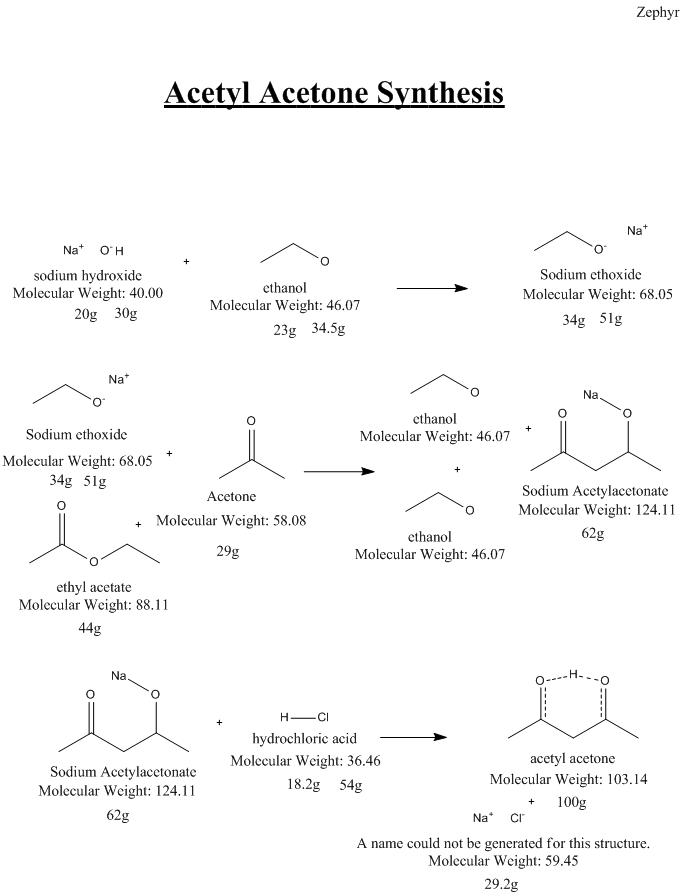
Insight on how it could be done better or issues with this route are appreciated.
|
|
|
| Pages:
1
..
43
44
45
46
47
..
77 |