| Pages:
1
..
42
43
44
45
46
..
66 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
From the air: the kerosene is exposed to air and is somewhat permeable to it. In proper glass ampoules that problem doesn't occur.
As a barrier against air oxygen, kerosene (and similar 'inert' solvents) is far from perfect.
[Edited on 5-11-2012 by blogfast25]
|
|
|
salmjak
Harmless

Posts: 2
Registered: 10-11-2012
Member Is Offline
Mood: No Mood
|
|
I've been looking at the reaction mechanism for this reaction during the day and this is what I came up with:
http://img84.imageshack.us/img84/4532/makingofpotassiumwithc...
It follows the path of a typical SN1-reaction
1) The OH-group leaves and a carbocat ion is left which KOH binds to.
2) Magnesium reduce the potassium and the hydrogen and leaves a negatively charged oxygen ion which will react with the now positively charged
magnesium ion.
3) The recently formed carbocat ion will react with the OH-group who left in the beginning and form the catalyst once again.
I'm new to this so please give me some feedback of which part of this mechanism that is realistic and which is not 
I suppose the reason a tertiary alchohol is used is because primary and secondary alchohols will go through a dehydration process and create water
which will in turn react with the potassium?
[Edited on 10-11-2012 by salmjak]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Most here believe the KOH forms an alkoxide with the alcohol. The K t-butoxide is then reduced to K and Mn t-butoxide, which hydrolyses to MgO and
t-butanol. More detail is provided much upthread.
See here and some more after that:
http://www.sciencemadness.org/talk/viewthread.php?tid=14970&...
No carbocation needed...
[Edited on 10-11-2012 by blogfast25]
|
|
|
salmjak
Harmless

Posts: 2
Registered: 10-11-2012
Member Is Offline
Mood: No Mood
|
|
Thanks for the reply and finding the older post for me! 
What I don't understand from those reactions is what's making the magnesium reduce the potassium? What is the force behind the behaviour?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Also explained higher up: the overall change in Gibbs Free Energy from left to right is negative, by about 200 kJ/mol. In simple terms it's really the
Heat of Formation of the MgO that drives the reaction.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Pok's method
So, I tried again. Miserable failure, again.
I decided to follow the original process by Pok, I followed it right down to the gram amounts. I decided to use a sand bath this time (which meant I
had to sneak to a nearby construction site to get sand, which isn't easy to find in the city apparently- unless you want a 75lb bag). My thinking was
that perhaps the sand creates a more even temperature that surrounds the flask and allows the K to coalesce together.
I always had a flask on the hotplate before and although the display shows the plate temperature it is not an accurate display of the reaction
mixtures' temperature.
Anyways, sandbath... glass tube cooled with wet coffee filter paper, timed addition of the t-butanol as per the original method, air kept out, bla bla
bla... nothing.
I wish I could post some successful pictures instead of failures which is becoming rather embarassing. This time the residue at the end had a tan
color, usually it is white. Weird.
Once again I am inclined to blame the solvent but this is the same solvent that gave me micro balls of potassium in previous attempts! Unscented,
deodorised lamp oil. I will try the Pok method again, tomorrow perhaps, with the famed "baby oil". I see profanity and frustrations ahead though.
I wish a member who got it to work lived close to me so that I could give them some of my KOH, Mg, t-BuOH and use their own solvent for fun, just to
see.
I am wondering though, is this solvent technically deodorized kerosene? I do have kerosene labelled "kerosene" but it is the one which smells of
diesel and is sold in the camping section of the hardware store.
Anyways:
The solvent:

Reagents mixed before solvent addition

The set-up

Stable temperature the whole time (4 hours)

Reaction after 3 hours

Beautiful non existant potassium. Residue does nothing with water, only the hiss of hot KOH 

[Edited on 20-11-2012 by Mailinmypocket]
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Mailinmypocket  | I wish I could post some successful pictures instead of failures which is becoming rather embarassing.
[...]
I am wondering though, is this solvent technically deodorized kerosene? I do have kerosene labelled "kerosene" but it is the one which smells of
diesel and is sold in the camping section of the hardware store. |
It's difficult for anybody here to help if
you don't post the experimental. Notably missing are the mass of the reagents and solvents, and a timeline of actions and temperatures seen.
As for the solvent, the original solvents were hydrogenated ones, removing aromatics and alkenes. Apparently the relative electron deficit may have
interfered with the reaction somehow.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
The reason I didn't post the exact masses and timelines is because I followed the procedure done by "Pok" originally, I still should have added them
to my post though. Ill edit that when I get a chance. A few posts up Blogfast quoted the proportions used in Pok's original preparation, those are the
ones I used.
As far as the timeline goes- I should definitely add that also, although there is essentially no difference compared to previous attempts except for
the fact that I never saw K, ever. Last time I did it I saw small balls immediately after the first alcohol addition.
[Edited on 20-11-2012 by Mailinmypocket]
|
|
|
Pok
potassium Prometheus
  
Posts: 176
Registered: 5-12-2010
Member Is Offline
|
|
I can imagine two reasons:
1. Maybe your lamp oil isn't suitable. It should have a boiling range comparable to that of Shellsol D70, which you can check by reading the MSDS.
Maybe there really is some stuff in your oil, that is poisonous for this reaction. I also used lamp oil from two different brands (which you will not
have in Canada) and they both worked perfectly (as good or even better than Shellsol D70!). But this doesn't mean that your lamp oil is equally good.
2. Your Mg is VERY coarse. The first step of the reaction (the evolution of hydrogen from water of crystallization in KOH with Mg) will even work
halfway with huge Mg pieces. Did you notice a strong H2-evolution at a moderate heat? But I'm not sure about the second step (the real reaction). Only
NurdRage's video shows very coarse Mg pieces, but not as compact as yours. The total surface area of your Mg is very small!
Most probably:
If your Mg is too coarse, not ALL of the H2O molecules in the technical KOH will be removed, because some parts of the KOH will never contact these
few Mg pieces. This is what I learned when I tried to do the first step with large Mg pieces. And thus your main reaction will not proceed because the
remaining water prevents the reaction! Even after 3 hours the KOH looks a bit transparent, a bit jelly-like. This is a hint that there is still water
in the KOH!
Try to file some Mg by hand or buy 100 µm size Mg powder.
If you don't have Shellsol D70, I think lamp oil is still the best choice and the kerosene is the worst choice.
[Edited on 20-11-2012 by Pok]
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Pok  | I can imagine two reasons:
1. Maybe your lamp oil isn't suitable. It should have a boiling range comparable to that of Shellsol D70, which you can check by reading the MSDS.
Maybe there really is some stuff in your oil, that is poisonous for this reaction. I also used lamp oil from two different brands (which you will not
have in Canada) and they both worked perfectly (as good or even better than Shellsol D70!). But this doesn't mean that your lamp oil is equally good.
2. Your Mg is VERY coarse. The first step of the reaction (the evolution of hydrogen from water of crystallization in KOH with Mg) will even work
halfway with huge Mg pieces. Did you notice a strong H2-evolution at a moderate heat? But I'm not sure about the second step (the real reaction). Only
NurdRage's video shows very coarse Mg pieces, but not as compact as yours. The total surface area of your Mg is very small!
Most probably:
If your Mg is too coarse, not ALL of the H2O molecules in the technical KOH will be removed, because some parts of the KOH will never contact these
few Mg pieces. This is what I learned when I tried to do the first step with large Mg pieces. And thus your main reaction will not proceed because the
remaining water prevents the reaction! Even after 3 hours the KOH looks a bit transparent, a bit jelly-like. This is a hint that there is still water
in the KOH!
Try to file some Mg by hand or buy 100 µm size Mg powder.
If you don't have Shellsol D70, I think lamp oil is still the best choice and the kerosene is the worst choice.
[Edited on 20-11-2012 by Pok] |
Thanks for the helpful reply - you definitely confirm some of my suspicions! The solvent itself, in my opinion, doesn't seem to be the cause of my
problems as I have had 1-2 mm diameter balls of K previously, I also have a very small amount of commercial K which I have stored under both solvents
(less than 100mg each test) and they both remained unchanged under baby oil and the lamp oil. The main advantage I have noticed with the lamp oil is
the reduced viscosity which would improve coalescence of the K. Baby oil is thicker.
The magnesium is definitely NOT what I was expecting when I purchased turnings. Despite the Mg being reagent grade turning for grignards it seems much
chunkier than other Mg I have seen for the same purpose- that's the problem with not seeing what you buy. I was expecting something a little smaller.
Oh well, live and learn I guess.
I will try and grind up some of this Mg tonight and see what happens, I just can't believe my bad luck! I have a ton of rolls of Mg ribbon, the stupid
"coated" granular Mg which didn't work, and now these nuggets of high quality stuff that are too big to work  Sitting on a huge pile of useless Mg. Sitting on a huge pile of useless Mg.
I will do a mini run and try and grind the Mg and see where that takes me.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
As a last ditch attempt I decided to try run and see what happens- the only difference is that once the dehydration of the KOH started I saw a lot of
K balls so I just added all the catalyst all at once.This is how it was 10 minutes ago, now there are four balls about 2-3mm in diameter. It is also being done with the baby oil!
**Just realized there is a god damn dog hair in the solvent... Animals...
[Edited on 20-11-2012 by Mailinmypocket]
|
|
|
condennnsa
Hazard to Others
  
Posts: 217
Registered: 20-4-2010
Location: Romania
Member Is Offline
Mood: No Mood
|
|
I too have failed with all attempts with this reaction.
I have tried with mg turnings from likurg, merck grignard mg, pyro magnesium, filed mg boiled anodes, filed mg pencil sharpeners. and many many
solvents. all attempts with t-butanol.
I concluded that the secret is the magnesium. It seems to me that it's not necessarily the purity in terms of % magnesium content, but that it's
certain impurities that people with the wrong magnesium have in higher percentage .
I have no idea what these elements might be, but with all my reactions the beginning is fine, I have steady evolution of hydrogen, which slowly but
surely dies down, and the magnesium turns dark.
You have to get lucky with the magnesium you get.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Hmmm… the large variety of magnesium grades tested could in fact also point to the possibility that it’s precisely NOT the magnesium that’s at
fault where failure occurs. One would expect at least one of them to work, wouldn’t one? As regards some ‘poison’ rendering the magnesium
inactive, it’s possible but you’d have to believe various sources differ greatly in trace elements accompanying the Mg across these grades.
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
Well, I ordered some Shellsol 
Not because I believe its the only solvent that will work, but I want to run some comparative tests since I have tried quite a few solvents and had
weird results.
Will report on the outcome when I receive it!
|
|
|
Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
This is so interesting thread. How about using solid parafine?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Small problem: it's solid! The problems with solid parafin have been discussed higher up...
|
|
|
Vinylogous
Harmless

Posts: 17
Registered: 19-10-2011
Location: 'Murcah
Member Is Offline
Mood: Vilsmeier-Haacking
|
|
Successful first potassium attempt!
My friend and I succeeded in making potassium last night, albeit an extremely small amount. Not too shabby for first attempt with sub-optimal reagents
and a terribly inaccurate scale.
Reagent table: (weights are very approximate, my best scale is a $30 digital scale from the big box store, error is +/- a gram at least. Volumes are
+/- 0.02 mL. The tricky-to-acquire reagents are a few months old, but otherwise seem to be of good quality. KOH might be a bit wet for my liking)
3g Magnesium powder, approx. 40-80 mesh, Sigma
5g KOH pellets, Sigma
1.8 mL t-BuOH, Fisher
50 mL Ultra-Pure Lamp Oil, Lamplight Farms (bp 250-280 degC), from Ace Hardware
Toluene, Kleen-Strip, Ace Hardware
A 100 mL round-bottom flask was charged with magnesium, lamp oil, and t-BuOH (0.2 mL), and fitted with reflux condenser, aluminum foil serving as air
exclusion. The mixture was brought up to 200 degC and held for 10 minutes. The surface of the magnesium seemed to become a bit shinier. The mix was
cooled back to 40 degC and KOH (5g, but it may have been more since my scale is terrible) was added.
The mix was brought up to 220-230 degC. Around 180 degC, gas evolution was observed, becoming quite violent around 190, and at one point my friend and
I observed a bubble of grey-blue gas (I have no idea what the heck that was) burst and dissipate. The solution went from clear to a mucky
yellow-brown, and then cleared up again. The bubbling settled down and temperature stabilized around 210-220 degC. The flask was charged with t-BuOH
(1.0 mL). The mix was offgassing small bubbles at a mild rate, which stirred up the KOH slightly, but overall there was not really any mixing.
Additional t-BuOH (6 x 0.1 mL) was charged, once every ten minutes over 1 h. The mix was held for an additional 20 min, then cooled to RT.
No potassium spheres were visible. A white gel-like muck had formed, with most of the magnesium powder still in place, with a darker grey patina. We
very carefully dumped a small amount of the reaction mix into a bucket of water in near darkness and witnessed numerous tiny sparks of light!
The next day, the mix had turned brownish. I broke up a solidified lump at the bottom of the flask, dumped the reaction mix into toluene (100 mL),
slurried, decanted most of the solution, leaving the gunk and powder behind, and charged more toluene (100 mL). The mix was heated to 80 degC with the
magnetic stirrer on. Nothing floated to the surface.
I'll be running again with perhaps a slight excess of magnesium, and also agitating the contents every now and then with a spatula to get better
homogenization.
[Edited on 25-11-2012 by Vinylogous]
[Edited on 25-11-2012 by Vinylogous]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Nice, but most of us here only achieve K globules of appreciable size after 3 - 4 h of heating at +/- 200 C.
|
|
|
Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
I will next week start this experiment 
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Just as a crazy idea, would a catalytic amount of iodine help kickstart the reaction by activating the magnesium? This could perhaps allow a quicker
better reaction between the magnesium and the tertiary alcohol. It could be worth a try...
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
Vinylogous
Harmless

Posts: 17
Registered: 19-10-2011
Location: 'Murcah
Member Is Offline
Mood: Vilsmeier-Haacking
|
|
I guess I need to run it longer as well. Thanks!
Quote: Originally posted by White Yeti  | | Just as a crazy idea, would a catalytic amount of iodine help kickstart the reaction by activating the magnesium? This could perhaps allow a quicker
better reaction between the magnesium and the tertiary alcohol. It could be worth a try... |
I have some. I'll give it a try next time I get a chance to run the reaction. I've got to look that combination up in Bretherick's first, I can see
that mix forming something saucy as a side reaction.
[Edited on 25-11-2012 by Vinylogous]
|
|
|
Mailinmypocket
International Hazard
    
Posts: 1351
Registered: 12-5-2011
Member Is Offline
Mood: No Mood
|
|
The amount of t-butanol you are using seems to be a tad high compared to other successful results used earlier on. My last attempt yielded many balls
of K, they just didn't last after 30 minutes.. but they did coalesce.
Just as a test, try this:
Add the Mg and KOH together in your flask, under the solvent. Ramp up the heat and when you see the reaction happening between the Mg and KOH, add the
entire dose of your t-BuOH diluted in 6ml of the same solvent. After doing this I have seen K appear almost instantly and last 15 minutes or so before
disappearing. I am wondering if adding the catalyst while the magnesium is reacting with the KOH somehow catches the Mg at a "perfect time". This may
be a clue as to why adding the catalyst at the beginning of the reaction also works, because it is present to start reacting as soon as the Mg starts
its vigorous reaction with the KOH @ about 140-170c.
I am just confused as to why it seems to be so difficult given the good reaction conditions
Also, I was having a much harder time when I was using a greater amount of t-butanol. Try 0.5-0.6 t-BuOH in enough solvent to make 6ml's. It's easier
to handle in larger volumes.
|
|
|
White Yeti
National Hazard
   
Posts: 816
Registered: 20-7-2011
Location: Asperger's spectrum
Member Is Offline
Mood: delocalized
|
|
Would linalool be a suitable substitute for tert but alcohol?
"Ja, Kalzium, das ist alles!" -Otto Loewi
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I've already asked myself that too. It's a good question because it's a tertiary alcohol too. The end group near the OH-group might cause steric
hindrance though.
Only one way to find out...
|
|
|
tetrahedron
Hazard to Others
  
Posts: 210
Registered: 28-9-2012
Member Is Offline
Mood: No Mood
|
|
then why not terpineol
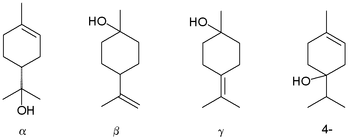
or other terpene alcohols (pine oil)? the alpha isomer is made from limonene (orange oil) or alpha-pinene (turpentine) [1][2].
|
|
|
| Pages:
1
..
42
43
44
45
46
..
66 |