| Pages:
1
..
38
39
40
41
42
..
81 |
Laboratory of Liptakov
International Hazard
    
Posts: 1386
Registered: 2-9-2014
Location: Technion Haifa
Member Is Offline
Mood: old jew
|
|
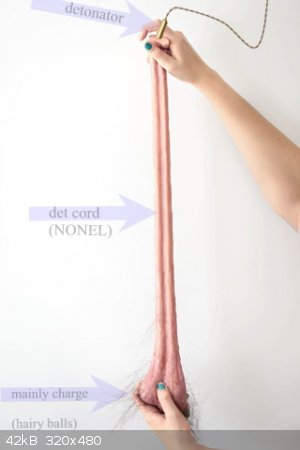
Of course, Hairy Balls exist: ...........
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Cheesy balls    the new sem(sex)tex... the new sem(sex)tex...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
James Ikanov
Hazard to Self
 
Posts: 81
Registered: 12-7-2015
Location: Alaska
Member Is Offline
Mood: Zen
|
|
Supposing that I felt the need to do something exceptionally stupid and make guanidine nitrate via the process described here:
http://www.google.com/patents/US4390726
IE, mixing it in a 1:3 ratio and melting it in the presence of silicic oxide, is there anything this patent DOESN'T mention?
Better yet, is there a method of using all this to make the nitrate without the inherent explosion risk of molten ammonium nitrate? It sounds like a
very interesting way to work towards a lot of the safer guanidine primary/secondary explosives... 
“To do good work one must eat well, be well housed, have one's fling from time to time, smoke one's pipe, and drink one's coffee in peace” -
Vincent Van Gogh
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by James Ikanov  | Supposing that I felt the need to do something exceptionally stupid and make guanidine nitrate via the process described here:
http://www.google.com/patents/US4390726
IE, mixing it in a 1:3 ratio and melting it in the presence of silicic oxide, is there anything this patent DOESN'T mention?
Better yet, is there a method of using all this to make the nitrate without the inherent explosion risk of molten ammonium nitrate? It sounds like a
very interesting way to work towards a lot of the safer guanidine primary/secondary explosives...  |
Did you really searched into the forum?
Compilation: Synthetic routes to tetrazole compounds based on OTC materials. by Engager...
Edit...
Oh yeah 1975 posts, my favourite year!
[Edited on 20-6-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
How would an azide mediated curtius rearrangement on a carboxyl group behave with two orho nitrogroup present on a benzene ring, for example 2,4,6
trinitrobenzoic acid? Just decarboxylation?
|
|
|
Armistice19
Hazard to Self
 
Posts: 87
Registered: 19-6-2007
Member Is Offline
Mood: Brain sponge activated!
|
|
Seems Legit
I met an older man not too long ago who claimed to be some kind of explosives expert of sorts for the U.S. military. He was very vague in his story
telling, and obviously attempting not to give too many details. He may have been legitimate, but who knows. I believe you should never trust any
single individuals word alone, especially when you consider that some civilians enjoy lying about being veterans for personal benefits. Either way, he
told me an interesting improvised safety technique that he said he was taught to use during his time in service. It seemed theoretically sound, but
not necessarily effective if ever actually applied. I was curious to hear some of the other forum members thoughts on the technique. Would it be
effective? Or perhaps it's just an emergency option, and is simply better than taking no precautions at all?
Here goes:
When working with energetic materials that were static sensitive, he said that (in order to properly ground out) one could hammer a piece of rebar
deep into the ground, and then pour salt water down the rebar so as to properly soak the earth that it penetrated. He would then say that at this
point one could wrap copper wire around their wrist, and then wrap the other end of the wire to the rebar.
My basic question here is, if this were to be actually applied, would it really actually keep you relatively safe from static discharge/accidental
detonation?
Thoughts? comments?
“Imagination is more important than knowledge. For knowledge is limited to all we now know and understand, while imagination embraces the entire
world, and all there ever will be to know and understand.” -Albert Einstein
|
|
|
NedsHead
Hazard to Others
  
Posts: 409
Registered: 9-12-2014
Location: South Australia
Member Is Offline
Mood: No Mood
|
|
I think it should keep you safe, provided you are wearing clothing made from natural fibres that won't produce any unwanted static, it sounds safe to
me. when I was handling ANFO explosives in an underground mine we would just put a knee on the ground and that was good enough
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
The rebar in ground with salt solution should be an effective grounding rod. If you did use it, I'd recommend a more sure connection of the wire to
the rod, rather than just wrapping it around the bar. Also, you should test the continuity between yourself and the rod, so there isn't a false sense
of security and you know it actually works.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by nitro-genes  | | How would an azide mediated curtius rearrangement on a carboxyl group behave with two orho nitrogroup present on a benzene ring, for example 2,4,6
trinitrobenzoic acid? Just decarboxylation? |
Normally it should work as usual thus leading to the TNA isocyanate (via TNBenzoic chloride and NaN3)
(O2N)3C6H2-CO-N3 -heat-> (O2N)3C6H2-N=C=O + N2(g)
Then after hydrolysis TNAniline
(O2N)3C6H2-N=C=O + 2 H2O --> (O2N)3C6H2-NH2 +(HO)2C=O (= CO2(g) + H2O)
Decarboxylation will naturally occur if you heat TNBenzoic acid at 100°C --> TNBenzene
For example this may happen in part while heating TNBenzoic acid with HN3
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
You want to DISSIPATE any charge slowly, rather than by dumping it is a big fat apark... So how about a nice big resistor in the circuit between your
body and the ground point?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Aurium
Harmless

Posts: 46
Registered: 4-10-2015
Member Is Offline
Mood: Energetic
|
|
Molecular sieves in Nitration?
Quick question from someone with little chemistry education.
"3A" Molecular sieves are often used to easily remove water from solvents.
Water contamination is what limits nitration Acid/Product ratio.
I wonder if Molecular sieves can be used to keep an H2SO4+HNO3 nitration mixture free from the water generated in the process of nitration.
Or just to remove water from 70% HNO3 to bring it up to fuming HNO3.
Why is this not possible?
Surely there is something I am missing here about the behavior of molecular sieves.
A quick google search reveals no answer to my questions.
Thanks for your answer!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Aurium  | Molecular sieves in Nitration?
Quick question from someone with little chemistry education.
"3A" Molecular sieves are often used to easily remove water from solvents.
Water contamination is what limits nitration Acid/Product ratio.
I wonder if Molecular sieves can be used to keep an H2SO4+HNO3 nitration mixture free from the water generated in the process of nitration.
Or just to remove water from 70% HNO3 to bring it up to fuming HNO3.
Why is this not possible?
Surely there is something I am missing here about the behavior of molecular sieves.
A quick google search reveals no answer to my questions.
Thanks for your answer!
|
MS may be used to take the water away and favourize/help nitration...there is a paper about HNO3/propionic anhydride and MS used to acheive 98%
dinitrotoluen...but I wonder what would be the difference with a little more HNO3/PA alone...
MS has the advantage of being solid and easily recovered by filtration and eventually recycled by heating.
I think that if you use granules of MS to dehydrate HNO3 or to help with nitration, it has to be a silica based one, not a carbon based because it
would otherwise oxydise.
When adding silica gel beads (a type of MS) into water they crack and are reduced into tinier silica pieces like sand...I guess the same if not worst
will occur with HNO3...at least it won't dissolve. So the beads shape will more likely be lost quite fast.
MS is used to dehydrate solvents but ones that doesn't contain too much or a lot of water...absorption ability is in the range of 13-17% water by
weight...
This means that to dehydrate efficiently 100g 69% HNO3 (=70.92 ml because d=1.410) you would need at least 200g MS...and this may be impractically
high...or not I don't know.
If you do the testing, I'm curious to read the results.
[Edited on 30-6-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Aurium
Harmless

Posts: 46
Registered: 4-10-2015
Member Is Offline
Mood: Energetic
|
|
I can't get any google information on the behavior of MS in acid, and I don't have access to all the online journals. ( I do, but holy hell 50euros
for a pdf betting on good info. )
Hum I get this feeling that somehow the charge of the positive acid outside vs the neutral water inside will limit the further diffusion of water
inside the beads, to the point of the MS being useless.
Worth a test anyway.
I'll use the silica based MS you recommend and try dehydrating 70% HNO3. If I succeed I'll try making ETN with MS swirling about.
I'm away from my lab ATM but I'll let you know the results in a month or something when I get the chance to test this.
[Edited on 1-7-2016 by Aurium]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
OK for the HNO3 concentration...maybe opt for cold distillation under vaccuum to recover as much HNO3 from the solid slurry of MS beads.
I would avoid too sensitive HE material (to shock, heat or friction) "with Molecular Sieves swirling about".
Friction between solid "sand" particles may give troubles in that case.
Edit:
Yeah 2016 posts  
[Edited on 1-7-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
tandpasta
Harmless

Posts: 30
Registered: 13-10-2014
Member Is Offline
Mood: No Mood
|
|
I don't think this is really a 'energetic materials' question, but I figured you guys would know best about this kind of stuff:
I frequently make camp fires and I was wondering about if there are any cheap ways of making colored flames for an extended amount of time. But it has
to stay 'safe', i.e. not more unsafe than a regular campfire. So no toxic combustion products, risk of spattering, etc. Ideally something that lasts
for say an hour and could make the flames different colors over time.
Any ideas?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by tandpasta  | I don't think this is really a 'energetic materials' question, but I figured you guys would know best about this kind of stuff:
I frequently make camp fires and I was wondering about if there are any cheap ways of making colored flames for an extended amount of time. But it has
to stay 'safe', i.e. not more unsafe than a regular campfire. So no toxic combustion products, risk of spattering, etc. Ideally something that lasts
for say an hour and could make the flames different colors over time.
Any ideas? |
The main problem is sodium (always present in trees, thus in natural wood) and carbon (also present by definition) that makes yellow, orange flames
and hide the usual colourizers...so colour expression will be bad even with hurge quantities...
You may get partial green flames if you throw an electric wire into the fire with its PVC coating...while burning the PVC sets HCl free then react
with Cu/CuO to make CuCl2 and CuCl (this last responsible of the blue colour...and blue+yellow = green).
But to get noticeable effect you need a lot of wire --> toxic fumes and HCl 
You could get much better effects by playing with cotton-cellulose whool soaked a few times into KCl then into demi water (to wash the Na(+) away)
then into aceton (to take the water away) and then into ethanol (burn-alcohol) or methanol.
The alcohol (ethanol or methanol) must be saturated with the colouriser salt/stuf and set into a heat resistant recipient (glazed ceramic is best):
Put a lot of the prepared cotton into it and use it as a fuse...
-CaCl2 --> orange-pink
-SrCl2 --> red
-CuCl2 -->electric blue-green
-LiCl --> deep red
-Trimethyl borate ester --> green
-Ba(OH)2+ BaCl2 --> pale green
This will last from 5 minutes to 30 minutes depending on the recipient shape, quantity of alcohol and surface of the burning fuse...
Once exhausted and flame off, replenish with clean fuel.
Beware that alcohol flame may be present but nearly invisible...so be sure it is off before adding the flamable fuel...otherwise your bottle may take
fire.
Not for kids without adult advice or care!
[Edited on 7-7-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Lotilko
Hazard to Self
 
Posts: 76
Registered: 9-6-2016
Member Is Offline
|
|
Highly energetic nitramines
I was reading about furazans and furoxans and I found this document. It's sadly in chinese, but it gives the densities and detonation velocities of
some highly energetic nitramines. I am primarily interested in HHTTD and compound 3, which appears to have a extremely high VOD and density. Could it
outperform ONC, or is this about theroretical preparation of these materials? I know the flourine atoms cause the high density, but also there is no
oxygen in the molecule. Maybe the lack of oxgen inhibits the formation of COF2, which would be a loss of fluorine so instead, HF forms
which has a relatively low mass. What are your thoughts on this? Sorry if there are any mistakes or errors, this is my first post and english is not
my first language. Any help will be appreciated, thanks.
Attachment: Synthesis and Properties of the Fused Aza-polynitrocyclic Compounds.pdf (609kB)
This file has been downloaded 622 times
|
|
|
Bert
|
Threads Merged
11-7-2016 at 09:49 |
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Lotilko  | I was reading about furazans and furoxans and I found this document. It's sadly in chinese, but it gives the densities and detonation velocities of
some highly energetic nitramines. I am primarily interested in HHTTD and compound 3, which appears to have a extremely high VOD and density. Could it
outperform ONC, or is this about theroretical preparation of these materials? I know the flourine atoms cause the high density, but also there is no
oxygen in the molecule. Maybe the lack of oxgen inhibits the formation of COF2, which would be a loss of fluorine so instead, HF forms
which has a relatively low mass. What are your thoughts on this? Sorry if there are any mistakes or errors, this is my first post and english is not
my first language. Any help will be appreciated, thanks.
|
Looks like some of the chemical pathways are purely theorical...or practically impossible (like joining a second cyclo penta-ring from a dibromo
compound itself generated by addition of Br2 onto a cyclopentene what is known to happen in trans addition (thus both Br atoms are not on the same
side of the pentaring plane...)).
Some pathways with the yields aside are plausible and probably inspired from related internationnal litterature.
Like many writing, the densities and VOD are exagerated or based mainly on thermodynamic calculations (much cheaper than actual testing and
measurements whitout the need to synthetise much material (testing may require a kilogram))...this effect is sadly true for all big HEM lab...like
Klapote's...
Now most follow the rule: "Publish or perish"...so quality of the work is less because they focus on the quantity.
SIDE NOTE:
Lately I have noticed that Klapote's team had made a mistake in most of their publications....they based their detonic calculations onto the density
of the molecule at the X-ray diffraction temperature (-100°C) what is not the normal use T°.
In fact by using the density at -100°C you overestimate the density vs at 20°C and all related detonics parameters are thus much higher and looks
sensational/promising...
Now since past year they have applied an automatic correction based on dillatation coefficient in a way to extrapolate the density of the crystals
from -100°C to the standard temperature... this might look a good idea to reduce the biased density value since contraction or dillatation may be
considered linear into a 100°C range (or more for certain compounds).
But it is not a good idea because:
1°) They use a single dillatation coefficient...while each compound and each specific crystaline form has a specific coefficient (sometimes dependant
on the axis ... thus anisotropic)
2°) Most compounds tends to srink and increase their density while cooling but it is not always the case!
3°) Some compounds change crystalline form by passing from -100°C to 20°C and this will affect the dillatation coefficient that may change
dramatically or even change sign (thus instead of srinkage you get dillatation)
So their calculation are purely indicative and quite uncertain.
About your fluorinated compound n°3, you said that there is no oxygen but you missed the point that it is only the amine skeletton on what you
perform the nitration to get a nitramine...and nitramine by definition is -N(-NO2)- and so it does contain 2 oxygen atoms!
Also perfluoromethyl (CF3-) would be as bad as teflon thus unreactive and inert...(except maybe with very reactive/reductive metals (Na, K, Li, Mg,
Al) with what it does make kind of thermites if well divised and homogeneously/intimately mixed)
You obviously confused difluoroamine energetic group (-NF2) which like related dihaloamino group (-NCl2, =N-Cl, -NBr2, =N-Br) in hydrocarbon skeletton
are energetic owing to the easy release of halogen and combination with the hydrogen as HF, HCl or HBr providing the driving force of the energetic
decomposition...the remaining C usually stays behind...as a black smoky cloud.
[Edited on 11-7-2016 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Lotilko
Hazard to Self
 
Posts: 76
Registered: 9-6-2016
Member Is Offline
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by Lotilko  | I was reading about furazans and furoxans and I found this document. It's sadly in chinese, but it gives the densities and detonation velocities of
some highly energetic nitramines. I am primarily interested in HHTTD and compound 3, which appears to have a extremely high VOD and density. Could it
outperform ONC, or is this about theroretical preparation of these materials? I know the flourine atoms cause the high density, but also there is no
oxygen in the molecule. Maybe the lack of oxgen inhibits the formation of COF2, which would be a loss of fluorine so instead, HF forms
which has a relatively low mass. What are your thoughts on this? Sorry if there are any mistakes or errors, this is my first post and english is not
my first language. Any help will be appreciated, thanks.
|
Looks like some of the chemical pathways are purely theorical...or practically impossible (like joining a second cyclo penta-ring from a dibromo
compound itself generated by addition of Br2 onto a cyclopentene what is known to happen in trans addition (thus both Br atoms are not on the same
side of the pentaring plane...)).
Some pathways with the yields aside are plausible and probably inspired from related internationnal litterature.
Like many writing, the densities and VOD are exagerated or based mainly on thermodynamic calculations (much cheaper than actual testing and
measurements whitout the need to synthetise much material (testing may require a kilogram))...this effect is sadly true for all big HEM lab...like
Klapote's...
Now most follow the rule: "Publish or perish"...so quality of the work is less because they focus on the quantity.
SIDE NOTE:
Lately I have noticed that Klapote's team had made a mistake in most of their publications....they based their detonic calculations onto the density
of the molecule at the X-ray diffraction temperature (-100°C) what is not the normal use T°.
In fact by using the density at -100°C you overestimate the density vs at 20°C and all related detonics parameters are thus much higher and looks
sensational/promising...
Now since past year they have applied an automatic correction based on dillatation coefficient in a way to extrapolate the density of the crystals
from -100°C to the standard temperature... this might look a good idea to reduce the biased density value since contraction or dillatation may be
considered linear into a 100°C range (or more for certain compounds).
But it is not a good idea because:
1°) They use a single dillatation coefficient...while each compound and each specific crystaline form has a specific coefficient (sometimes dependant
on the axis ... thus anisotropic)
2°) Most compounds tends to srink and increase their density while cooling but it is not always the case!
3°) Some compounds change crystalline form by passing from -100°C to 20°C and this will affect the dillatation coefficient that may change
dramatically or even change sign (thus instead of srinkage you get dillatation)
So their calculation are purely indicative and quite uncertain.
About your fluorinated compound n°3, you said that there is no oxygen but you missed the point that it is only the amine skeletton on what you
perform the nitration to get a nitramine...and nitramine by definition is -N(-NO2)- and so it does contain 2 oxygen atoms!
Also perfluoromethyl (CF3-) would be as bad as teflon thus unreactive and inert...(except maybe with very reactive/reductive metals (Na, K, Li, Mg,
Al) with what it does make kind of thermites if well divised and homogeneously/intimately mixed)
You obviously confused difluoroamine energetic group (-NF2) which like related dihaloamino group (-NCl2, =N-Cl, -NBr2, =N-Br) in hydrocarbon skeletton
are energetic owing to the easy release of halogen and combination with the hydrogen as HF, HCl or HBr générâtes the driving force of the energetic
decomposition...the remaining C usually stays behind...
[Edited on 11-7-2016 by PHILOU Zrealone] |
Thanks for clearing that up, PH Z. 
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
Hi!
I'm trying to get pure ammonium nitrate from an instant cold pack which contains calcium ammonium nitrate (CAN). Tha chalck was easily filtered, but
it also contains a wax coating and I don't know how to get rid of it. With hexane or similars maybe?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
http://lmgtfy.com/?q=sciencemadness.org+ammonium+nitrate+Cal...
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
kratomiter
Hazard to Others
  
Posts: 106
Registered: 30-9-2012
Member Is Offline
Mood: No Mood
|
|
That doesn't solve very much... The calcium was not an issue.
But I didn't found any way to isolate AN from the wax (in fact guar gum or any similar hydrosoluble polymer, not actual wax). Guar gum concentration
is lower than 2% AFAIK, maybe it could be used as an ANFO-like explosive.
Anyway, if I found something useful I'll post results.
|
|
|
dangerous amateur
Hazard to Others
  
Posts: 148
Registered: 8-7-2011
Member Is Offline
Mood: No Mood
|
|
Ammonium nitrate + metal powder: is this going to react
Hi guys,
in pyrotechnics evrybody knows that for example potassium nitrate and aluminium or magnesium powder can be a dangerous combination, in presence of
moisture this can heat up to the point of self ignition
I wonder how ammonium nitrate would react under these conditions. Or rather if it should react at all.
Zinc powder will react, thats for sure. But I combined moist ammonium nitrate and fine aluminium several times now and I never had problems.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
Ammonal was an older explosive consisting primarily of NH4NO3 and Al powder ─ requiring a booster for its detonation!
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
In general, any nitrate and aluminium combination needs a stabiliser. Usually boric acid I think. Nitrate can be reduced to ammonia, and this
attacks the aluminium.
Magnesium isn't involved in a runaway reaction I know (it's alkaline stable), but it's so reactive anyway I think you need to coat it just to keep it
fresh.
|
|
|
| Pages:
1
..
38
39
40
41
42
..
81 |