| Pages:
1
2
3
4
5 |
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
MDA is unusual in its actions also as MDA and MDMA do not appear to form a cross tolerance with each other suggesting different modes of action in
ones body. Most methyl amphetamine variants do display a marked decrease from the amphetamine counter parts and this maybe where the confusion is
arising from but n-methyl- amphetamine and MDMA appear to show a marked increase deviating from the normal patern that many substituted amphetamines
show when methylated.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
-TheMadMen-
Harmless

Posts: 29
Registered: 29-5-2009
Member Is Offline
Mood: No Mood
|
|
Maybe I should change my name to sunlight. The only reason I called myself "TheMadMen" was because I'd be thought of that way by everybody on a forum
like this. But thank you, starman, for your nice greeting. Considering that you live in Western Australia, I am even more impressed, as do I. :-) So
hopefully the name "TheMadMen" would not apply after all. :-)
Okay, in my first address, I stated that amphetamine is more potent than methamphetamine, and I stand by this statement for many reasons. How many of
you have actually tried amphetamine, d-amphetamine, the dextro isomer, in so far as opposed to d-methamphetamine, or the "ice" and "speed" we all know
of?
The truth be known, there isn't actually any noticeable difference in potency in either compounds, most people cannot tell the difference, they are
equally fine in there effect, but amphetamine is more potent and lasts longer. The reason it does not turn up all over the place is because its more
difficult to synthesize(not really, it's still piss easy, but most people, unbenounced to me, prefer to go through a whole hassle of unnecessary
intermediates and steps to get a pathetically unworthy methyl group attached to the molecule). And of course, in the past 10-15 years, when the
"pseudoephedrine kitchen bench top cooks" turned up, the only choice they had was to synthesize methamphetamine, as they couldn't just lead to
amphetamine directly with one of there ""teqs"" starting from ephedrine or pseudoephedrine alone.
And a methyl group, in other compound families, often downgrades the potency, as is the case with codeine for instance, methyl-morphine. Makes it 1/3
or 1/10th less potent, I forget?
Either way, you get what I'm saying, I hope. And usually a methyl group does nothing to alter the function of the compound's primary function, as
would be the case with methylenedioxy ring on the amphetamine molecule, usually all a methyl group does is downgrade the potency, as I have described
above, and I'd speculate that the main reason for this is because of bodily metabolism more than anything. The methyl group seems to be a target of
many 'an enzymes contained within our bodies.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
This is a chemistry forum and not a lounge for wannabe srug cooks.
If drugs are what you want to talk about, please moey on ober to WetDreams or some similar haunt.
Sic gorgeamus a los subjectatus nunc.
|
|
|
setback
Hazard to Self
 
Posts: 50
Registered: 17-5-2009
Member Is Offline
Mood: No Mood
|
|
I think drug discussion that isn't blatant cookery, "swimming", or other idiocy is fine. I believe that is the policy on this site as well. Besides,
have you read the organic forum lately? Sure, there is non drug chemistry discussed, but there is far worse than this.
[Edited on 31-5-2009 by setback]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
setback: Swim'ing is not allowed on this site. Common sense usually dictates what a posters motive is; whether they have the intention of "cooking" or
not. Then appropriate action is taken. Spend a little time in detritus and see what I mean.
|
|
|
starman
Hazard to Others
  
Posts: 318
Registered: 5-7-2008
Location: Western Australia
Member Is Offline
Mood: No Mood
|
|
Well I would have thought any discussion on structure activity relationships of any compound would be acceptable to forum members.Lets not go
overboard with political correctness.The reason why compounds behave as they do....Isn't that at the very heart of chemistry?
Lets not not assume the worst of anybody who raises a question in the pharmacological arena.
Chemistry- The journey from the end of physics to the beginning of life.(starman)
|
|
|
-TheMadMen-
Harmless

Posts: 29
Registered: 29-5-2009
Member Is Offline
Mood: No Mood
|
|
Sauron, read my first post, drugs are not what i want to talk about, and i said i was amazed that people on this forum do want to talk about drugs. So
i thought, why not, and went on in and starting engaging in conversation with seemingly like-minded people about these kinds of topics.
| Quote: | | MDA is unusual in its actions also as MDA and MDMA do not appear to form a cross tolerance with each other suggesting different modes of action in
ones body. Most methyl amphetamine variants do display a marked decrease from the amphetamine counter parts and this maybe where the confusion is
arising from but n-methyl- amphetamine and MDMA appear to show a marked increase deviating from the normal patern that many substituted amphetamines
show when methylated. |
Hmm, thought provoking, perhaps, i admit that I've never researched into the interlated-dyanmics of methylated and umethylated amphetamine analogs, or
the cross-tolerance that they do not induce, nevertheless, i still fail to see how a methyl group could alter the primary pharmacological action in
any way other than to dim it down, as is the case with codeine, as i have described above. Methyl groups do not seem to bind to receptor sites
differently, they just actively dim the effect profile down, because maybe more of the drug is removed and metabolized by the body before it can reach
the brain etc....
In any case however, from a sociological perspective, let me tell you, methamphetamine is THE amphetamine, that everybody knows about, the most
infamous one, but it's also the less potent than amphetamine, that's all i can say, and at the end of the day it is true, believe me or not, or not
even less potent, but just as potent, it certainly seems to last longer however, double-blind placebo tests have documented that hardly anyone can
tell the difference between a dose of 10mg d-amphetamine and 10mg d-methamphetamine, so explain that?
[Edited on 31-5-2009 by -TheMadMen-]
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by -TheMadMen-  | | How many of you have actually tried amphetamine, d-amphetamine, the dextro isomer, in so far as opposed to d-methamphetamine, or the "ice" and "speed"
we all know of? |
Not the pure enantiomers, but I tried the racemates of both. Granted, in a completely uncontrolled setting and at a way higher dosage than 10 mg, but
I found the opposite to be the case: the N-methyl analogue is gentler but lasts distinctly longer (as in no sleep, not as in euphoria). In the opinion
of a colleague "you can't mistake one for the other", but it's not unlikely that this is placebo effect.
PS: over here plain racemic amphetamine is the most common form found on the street.
|
|
|
-TheMadMen-
Harmless

Posts: 29
Registered: 29-5-2009
Member Is Offline
Mood: No Mood
|
|
| Quote: | | but it's not unlikely that this is placebo effect. |
Exactly mate. Placebo is the word of utmost importance here. Even a compound as potent as amphetamine/methamphetamine can be swayed to a certain
extent by placebo, more people who take meth do indeed seem to expect it to be more "smooth" than normal amphetamine, so as a result it may feel that
way, for no other reason than ones placebo cognitive inhibitions however.
But bear in mind, I've never actually tried racemic amphetamine, racemic methamphetamine i have, which may explain why i have always found pure
d-amphetamine to be more potent. Who knows. I'm amazed that plain racemic amphetamine is the most common form of crank found on the street where you
live. Thats a good thing though, it's about time that people learned the truth, even junkies.  Here it is definitely meth, but that is soon about to change it seems. Here it is definitely meth, but that is soon about to change it seems.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Without going to far off topic in a threed about THEORETICAL amphetamines I have found some papers that seem to support your hypothesis and where as I
in no way shape or form agree with them I do not argue with data. It seems that in females the strength is alot more potant for both forms and that
males appear to have a quick peak in potancy but AMPH last and extended period of time.
Without going to far into my past history of drug use I can say that I was perscribed to dl-amphetamine sulfate for years and I personaly feel that
the methylated derivative is more potant and way smother then the material that I was perscribed to which lead to shakes and jitters as though one has
drank to much coffee. There is a also a noticable fog that ones head is in that is not there for the methyl form. This could be due to what the one
text states about METH in that it does not raise GLU levels where as AMPH has a large spike in it.
Mind you these text are based on locomotive basis and not the strenght the material binds to the receptors
Differences between d -methamphetamine and d -amphetamine in rats: working memory, tolerance, and extinction
| Quote: |
Abstract
Rationale Previously, we have shown that d-amphetamine (AMPH) was more potent than d-methamphetamine (METH) at increasing extracellular levels of
dopamine (DA) in the prefrontal cortex (PFC) at doses that had similar effects in the nucleus accumbens. Since working memory depends on PFC DA, it
was postulated that AMPH would also be more potent than METH at affecting working memory.
Objective To determine if AMPH is more potent than METH at affecting working memory.
Methods Working memory was measured in adult female Sprague-Dawley rats using a delayed-alternation T-maze task with multiple delays (1, 10, 60 s)
and food rewards. The percentage of food rewards consumed was also recorded. Animals were tested with METH and AMPH before and after a chronic
protocol, with measurements of locomotor activity used to test for pharmacological tolerance or sensitization. The effects of METH and AMPH on
extinction were also examined by omitting the food rewards from the T-maze.
Results Both METH and AMPH produced dose-related bimodal effects on working memory at the intermediate delay (10 s); however, AMPH was more potent
than METH. Both METH and AMPH initially also decreased the percentage of food rewards consumed in the T-maze. After chronic testing, animals displayed
tolerance to both the working memory impairments and the reduction in food reward intake produced by AMPH. Animals did not display significant
tolerance to the effects of METH on food reward consumption and performed worse in the T-maze after chronic testing. METH, but not AMPH, interfered
with extinction.
Conclusions These results indicate that METH and AMPH differ in altering working memory and the expression of tolerance, perhaps due to differences
in behavioral inhibition. |
Reference:http://www.springerlink.com/content/jdppjuv480ueb7xv/
http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=18...
As stated before this is a threed for theoretical amphetamine variants and mounds of information can be found thru a quick web search.
But I will attach a picture of a substance I have intrest in because a complete search of PubMed has turned up not a single bioassay of the substance.
As you can see it is nothing more then an indole variant of the bromo-dragonfly compound and it includes all the prerequisites for receptor binding
such as two singlet hydrogen doners and a reqion of steric occlusion just like the Bromo-dragonfly molecule.
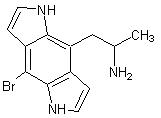
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
-TheMadMen-
Harmless

Posts: 29
Registered: 29-5-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for posting that article Sedit, it definitely seems to agree with my statement that amphetamine is more potent than it's methylated analogue.
But like you said, even yourself would say that on a subjective basis, you found methylated amphetamine to be more appealing on many levels.
If I read it correctly, amphetamine induces more dopamine release than methamphetamine? In rats. Surely that would be the calling card for such a
claim as I would make as to the superior strength of amphetamine to methamphetamine on a physiological basis? But again, subjectively, that may not be
the case!
In regards to the bromo-dragonfly, I admit I never really heard of it, but if it has a potency similar to LSD, then I am instantly fascinated by it,
I've never really heard of a compound having such a potency, or infact, clonidine, I think, not a psychedelic substance, but I think it is still dosed
on the microgram level. Seems to be a drug that comes to mind in that regard. Oh, and fentantly, and the super potent opiate analogs. But what the
hell, i am veering off course here immensely, forgive me, we are talking about theoretical amphetamine variants hehe, not clonidine or opiate analogs.
But anyway, yeah, I'm stunned at the bromo dragonfly. I Admit. Seems interesting.
[Edited on 31-5-2009 by -TheMadMen-]
[Edited on 31-5-2009 by -TheMadMen-]
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Sedit: I see no reason why the structure you've drawn would be a good candidate. Nitrogen is not an isosteric replacement for oxygen here.
Personally, I wouldn't take that. Aromatic amines are generally not so good for you. I'd like to discuss more the SAR aspects, which no one has
really brought up.... what about bromo-dragonfly lends well to increased potency. Bulk? Non-polar interaction? Secondary
pharmacophore (if so, what is it?)
How much work, beyond old-school SAR, has been done with the 5-HT2A receptor?
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Arrhenius I would not take it either because Bromofly compounds have a bad habit of causing vascular constriction sometime days or weeks later leading
to gang green and loss of extremities. That being said read the paper I posted on the page before this one and it will explain why I feel this would
be a good candidate for receptor binding. The oxygens on the furan rings donate a hydrogen to the receptor and lead to a strong interaction with the
binding site.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I've never heard that. In fact I find it hard to believe (not saying I don't trust you, I just don't trust lots of stuff on the net). On that note,
however, aren't all amphetamines and relatives a bit hypertensive? I guess I don't see a good reason why a drug can have real adverse effects
a week later.
Yes, I realize that the Br-dragonfly probably benefits from H-bonding at the furan, but in my experience :NH is a much poorer H-bond donor. I would
not be surprised if its pyrrazole analog is not very potent.
It has somewhat been brought up earlier, but how active would the purely steric analog be? This is comparable to the indane structures which have been
discussed. I would not be surprised if this is more active than amphetamine purely due to hydrophobic interaction. I'm not under the impression that
the choice of 4-halide should matter, since it's blocking hydroxylation and realistically has minimal ADME impact. This will probably suffer from high
protein binding, but give clues as to the value of furan:
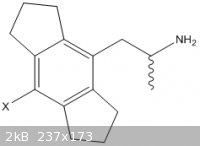
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
From Wiki of all places,
| Quote: | Toxicity
Pink ABDF powder.The toxicity of Bromo-DragonFLY is unknown for humans however at least three reports of death believed to be resulted from
Bromo-DragonFLY have been reported in Norway[3], Sweden[4] and Denmark[5][6]
Also, a Swedish man had to have the front part of his feet and several fingers on one hand amputated after taking a massive overdose. Apparently the
compound acted as a long-acting efficacious vasoconstrictor, leading to necrosis and gangrene which was delayed by several weeks after the overdose
occurred. Several other cases have also been reported of severe peripheral vasoconstriction following overdose with Bromo-DragonFLY, and a similar
case is also known from DOB. Treatment was of limited efficacy in this case although tolazoline is reportedly an effective treatment where
available.[7][8]
Overdoses, disturbing experiences, and Bromo-DragonFLY associated health problems have been described. One case in 2008 in England involved inhalation
of vomit, causing nearly fatal asphyxia.[9]
|
I have many more papers that I will look for later tonight. The problem is the vascular constriction does not go away quickly and after an
extended period of time for lack of blood flow to the extremities causes gangrene to set in and causes necrosis of the tissue.
I have not seen any synthesis of the compound you mentioned but I will keep an eye out as I look around later.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
I haven't either, and it surely wouldn't be easy. The published syntheses for dragonfly & hemidragonfly analogues are not exactly simple either.
I saw that on wikipedia, but my issue is that I'm not sure I trust Erowid. The resources available to Erowid are limited, and the forensic evidence
is too. I find the gangrene case to be extremely peculiar and not representative.
Also, all 'reported' (near)fatal hypertension seems to be as a result of overdose. Is this a good way to measure safety? I should think safety would
be better gauged by the number of safely consumed *reasonable* dosages.
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
It is quite probable that a lot of these compounds may have been prepared but never made it into the published literature.
Quite a lot of chemistry never gets published as there is nothing particularly original about it or the compounds have no interesting properties.
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Haha. People publish uninteresting stuff all the time!!  But very little gets
published relating to psychopharmacology now anyway. Some of the questions posed above would be considerations for a modern chemist doing 3D QSAR,
but probably not for Dr. Shulgin & co. who did purely homologation (somewhat randomly). But very little gets
published relating to psychopharmacology now anyway. Some of the questions posed above would be considerations for a modern chemist doing 3D QSAR,
but probably not for Dr. Shulgin & co. who did purely homologation (somewhat randomly).
|
|
|
ScienceSquirrel
International Hazard
    
Posts: 1863
Registered: 18-6-2008
Location: Brittany
Member Is Offline
Mood: Dogs are pets but cats are little furry humans with four feet and self determination! 
|
|
Good point, there are lots of useless and boring publications out there.
I have had to look up compounds where I was sure that there would be lots of homologues, only to find obvious and easy substitutions not listed in the
literature.
I am sure they have been made but never made it out of the world of theses and internal reports etc.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
I retract what I said before about nitrogen being a substitute for oxygen because I misunderstood what I read before and the hydrogen bond doner is
comming from the receptor and not the substance itself not only that but the whole charge of the area would be all wrong and quite possibly just repel
the receptor site. With that being said what about a sulfur analog? The area of influence for accepting the hydrogen bond would be greatly increased
and as long as the size is not to large possible create a stronger binding the oxygen.
For those of you who haven't taken the time to read the paper I uploaded(shame on you) I will attach the receptor conditions here, Also is a picture
of the difference in area of interaction that sulfur would cause

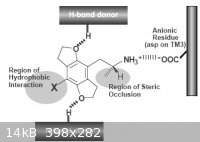
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Eh... I don't want to sound like a downer, but that's an utterly oversimplified model. "H-bond donor" is rather ambiguous. Did you draw that? One
would love to think it binds like that, but why should that be a multitude more potent than 2C-B?
Sedit: Do keep in mind that a :NH lone pair can act as a hydrogen bond acceptor, but not really when conjugated in a ring,
e.g. Sulfur is an isosteric replacement for oxygen, but it doesn't usually make much sense, as it's oxidized to the sulfone pretty readily by CYPs.
The only paper I see is the one you linked on female v. male rats. That one?
Seeing as how the duration of bromo dragonfly & friends seems to be rather long, I would be interested to see the duration of the 4-H phenyl
analogue. This is an intermediate, but I can't find any bioassay.
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
No the paper is page or so back that I got that picture of the binding mechanics from. Its not attached, its in a link in one of my post. Im sure it
is a highly over simplifyed model and the text goes into more details but that is the jist of why this binds so strong to the receptors.
IIRC the reason its so much more potant the 2C-B is because the closed ring structure prevents rotation of the oxygen bonds fixing it into a stable
position. This has been used in other variants of amphetamines also to attach a cyclopropane to the "tail" to stiffen it and enhance potancy greatly.
Over at blacklight you will find information about that substance and alot more then I could possibly ever know about this one also.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
Yes, I'm familiar with the cyclopropyl derivatives (which are probably legal). I'm not really a forum surfer; you won't find me elsewhere. I don't
care to read forums strictly about drugs, since it's the chemistry I'm interested in. I would propose that a larger molecule might be higher potency
simply because of hydrophobic interaction in the receptor binding pocket. Researchers often overlook the enthalpy gained by displacing a few water
molecules (also incredibly difficult to calculate). Hence, I would love to see the steric analog i brought up. I think for this reason some of the
pyrrole derivatives mentioned earlier will not work.
Personally, I'd like to see more experimentation on the aliphatic end of these molecules. Clearly phenethylamines and tryptamines can accept a wide
variety of N-alkylation. A derivative of LSD exists in which all but the indole ring are opened to give a long chain. I can only presume LSD-25 is a
highly privileged structure, given its potency. There is a very good chance that the molecule hits a secondary pharmacophore of the 5-HT receptors.
What do you think? Start here, or the indole.
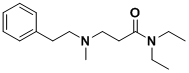
Ah... I'm sorry for straying off topic, this isn't an amphetamine.
[Edited on 2-6-2009 by Arrhenius]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
That topic was already discussed some time ago: http://www.sciencemadness.org/talk/viewthread.php?tid=8120
|
|
|
Arrhenius
Hazard to Others
  
Posts: 282
Registered: 17-8-2008
Location: US & A
Member Is Offline
Mood: Stochastic
|
|
That's a different molecule, but thanks, I hand't seen that thread.
|
|
|
| Pages:
1
2
3
4
5 |