| Pages:
1
2
3
4
5
6
..
60 |
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
| Quote: | "My plans
On the Sunday I plannig to prepare P from H3PO4 + C = H20 + CO + P.
I have real 600-650C in my glove
Good luck
Flayer
Sorry for my bad English."
by Flayer.
This was his first and last post at MSDB |
Kind of chilling (although according to his profile he was active 5 days ago). Phosphine was a major problem for most of my attempts, but luckily
(kind of) lots of it was generated with appreciable diphosphine to render it spontaneously flammable. Great idea there about oxidizing the phosphine
using CuCl2 or other salts. Might have to give that a try, usually this method is done by taking very high strength phosphoric acid, 95%+ and
kneading in saw dust then heating the resulting doughy mass.
[Edited on 5/31/2004 by BromicAcid]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
What would stop the P from giving the phosphides, if this works? Also, H3PO4 will not react as mentioned. Obviously it will not be in that form at the
temperatures C reduces at. Even so, the reaction 4H3PO4 + 16C = 6H2 + 16CO + P4, is all over the literature, so it ought to work, somehow. But I
haven't done it, so I wouldn't know for sure.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Orthophosphoric acid is less than ideal for a lot of reasons.
Dehydration produces pyrophoric acid, and then metaphosphoric acid, but this tends to volatalise before the temperature is reached where reduction by
carbon starts. I speak both from lit and experience.
Aside from the lead phosphate process, the most promising 'low temperature' carbon method I can find starts with calcium dihydrogen
othophosphate. This is used as a fertiliser as 'triple superphosphate', strong heat converts this to calcium metaphosphate, this when
reduced by carbon produces phosphorous and neutral calcium orthophophate. Its still a white heat process though.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Was looking at espacenet last night for P pats. that I could use. There are a lot of patents for sure and really only looked at a few. Someone with a
lot of time on their hands could find better than these, and if they speak German, even more. Click on the "requested patent" #'s.
The microwave patent Polverone mentioned:
http://l2.espacenet.com/espacenet/viewer?PN=US6207024&CY...
The or a Pb phosphate/H patent:
http://l2.espacenet.com/espacenet/viewer?PN=US4287165&CY...
An iron or Al/H3PO4/silicic acid/700C pat:
http://l2.espacenet.com/espacenet/viewer?PN=GB320598&CY=...
Different, but similar, silicon and ferrophosphorus, to illustrate somewhat of a variation of the above patent:
http://l2.espacenet.com/espacenet/viewer?PN=US1836618&CY...
PClx directly from phosphate at 700C with Cl:
http://l2.espacenet.com/espacenet/viewer?PN=US1926072&CY...
This one is at 1250C, sort of in the usual thermal way, with variations and great experimental lab detail:
http://l2.espacenet.com/espacenet/viewer?PN=US2897057&CY...
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Today I tried to make some elemental phosphorus, using trisodium phosphate as a fine powder (all ground by hand, my hand hurt for a while, I must be
holding the pestle wrong or something), fine silica, and aluminum, in the form of snipped up wires. There was an excess of Al because I figured it
was the reactant that would get mixed and used the most inefficiently.
I heated about 50 g total reactants in the distilling apparatus described in my furnace thread for about 2 hours on "hellfire"  . (the part of the apparatus in the furnace was glowing reddish orange. The only
difference from the apparatus I used than the one shown in that thread was that instead of bubbling the exit gasses through a tin can soldered on,
which I tried but wouldn't hold water, I put another 90 deg elbow on the end of the pipe and a short section pointing upwards, this part was
filled with water. . (the part of the apparatus in the furnace was glowing reddish orange. The only
difference from the apparatus I used than the one shown in that thread was that instead of bubbling the exit gasses through a tin can soldered on,
which I tried but wouldn't hold water, I put another 90 deg elbow on the end of the pipe and a short section pointing upwards, this part was
filled with water.
As the thing was heated, phosphine (so I think) started coming out of the end as a white mist, so I burned it off with my propane torch, it made
popping sounds and the mist disappeared.
After this, the water started getting milky, so I figured there was some phosphorus in there, but at the very end of the run, I heated the water up
until it boiled, and then dumped what I supposed would be a water/phosphorus mix into a tin can filled with water. All that came out was water.
Since the furnace ate a handful of wood or two every few minutes, I had to stoke the fire a LOT, and the only way to add more fuel was to take off the
lid, set it down on some bricks, add more fuel, and then put the lid back on. Every time I did this some of the water spilled out. I don't
think phosphorus is a good grass fertilizer.  The furnace is still cooling down
(I also fired some pottery) which takes about 5-10 HOURS! Thusly, I cannot check for more details. The furnace is still cooling down
(I also fired some pottery) which takes about 5-10 HOURS! Thusly, I cannot check for more details. 
The most "scary" part of the whole procedure had nothing to do with the reaction...
So I was sitting there watching the 30 cm high flames scream out of the furnace lid as glowing ash flew around me like snow (I had protection on)
mostly in view of about 3 neighbor houses, and all of a sudden, a FIRE TRUCK comes barreling down my street! You can guess what I was thinking. The
truck came closer to my house, I saw firemen suited up to do battle with their enemy, and they saw me suited up to do battle with my enemy, the truck
slowly screeched to a halt in front of my house. I started thinking, WHICH LOUSY NEIGHBOR TURNED ME IN?!,
Then the firetruck left and kept going!
It turns out they come once a year to adjust fire hydrants. There is a fire hydrant near my house  WHEW! WHEW!
Three things I have learned
1 do not stand on top of a thin piece of wood on top of a bucket with a saw in one hand and stomp on the wood to break it in half.
2 take altoids before putting on gas mask. 
3 making phosphorus might not be as easy as it sounds. 
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
| Quote: | | As the thing was heated, phosphine (so I think) started coming out of the end as a white mist, so I burned it off with my propane torch, it made
popping sounds and the mist disappeared. |
I got the white mist too at parts, I believe this is phosphorus pentoxide, it might have also been phosphorus vapor (too much furnace heat and too
short of a condenser may have made most of the phosphorus resist turning to a liquid resulting in it staying in the gas phase and oxidizing on exit.)
Plus there may have been some phosphine/diphosphine in there, I knew I had some form of phosphine because the bubbles were almost exploding when the
exited the water.
| Quote: | | After this, the water started getting milky, so I figured there was some phosphorus in there, but at the very end of the run, I heated the water up
until it boiled, and then dumped what I supposed would be a water/phosphorus mix into a tin can filled with water. All that came out was water.
|
What color was your final water, for some reason my pipe corroded at the water interface and resulted in a nasty orange color. What was the purpose
of heating the water till it boiled? To get the phosphorus to condense into single blobs?
| Quote: | | I don't think phosphorus is a good grass fertilizer. |
Actually, when I did a similar experiment and clouds of P2O5 rode across the land, a few weeks later there were mysterious dark green spots that
coincidentally ran across the same areas phosphorus clouds had roamed a few weeks before.
Personally I think the most reveling thing you could do is look at the contents of your reaction vessel. Being that the aluminum was not finely
divided it may have reated on the surface forming a thick Al2O3 coating which held in the molten aluminum and prevented further reaction. That is the
best I can come up with as to why this does not seem to have worked well. Should be easy to tell if you look at the reaction cake.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Some of this stuff has been covered allready. Trisodium orthophosphate is not a good candidate. Sodium dihydrogenorthophosphate, or a mixture of
trisodium and phosphoric acid or ammonium hydrogen phosphates that will get you sodium dihydrogen phosphate should work much better, as the sodium
metaphosphate this will form in the slow heating phase (you need a slow heating phase to dehydrate and avoid phosphine formation) reduces much more
easily.
I would not add silica to this. Under best conditions with carbon this would only leave the trisodium othophosphate as the product, with aluminium
expect some phosphides but you want the mixture to stay put while it reduces rather than form oxides and acids of phosphorous that distill out. I
think like bromic this is the mist you saw. Metaphosphoric acid itself is not a good candidate because its too volatile for carbon. Maybe aluminium
would would work but if you stick to a salt of metaphosphoric acid you stay in more documented chemistry. The only problem I can see if that if the
aluminium is active at the temperature of dehydration this will reduce the yeild.
Phosphorous is an oxidising agent too, excess aluminium favours phosphide formation. The majority of the flammable gas should be CO. If you have
phosphine it means you have hydrogen and something isnt being done right.
I'm not convinced wood will get the same sorts of temperatures as charcoal. I might be inclined to try a reduction with carbon using a charcoal
or coke run. Aluminium wire cant be cost effective. I also think you need to scale up. 50 grams total reactants is nothing, this isnt a reaction
you can do well small scale.
Edit, something else occured to me. What stops the aluminium reducing the silica instead?
[Edited on 27-8-2004 by Marvin]
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
PHOSPHORUS!!!
Ok, I did make some phosphorus.  But last night I confused it with
campbell's mushroom soup concentrate. But last night I confused it with
campbell's mushroom soup concentrate. 
Right after the fire truck came, I decided to stop the reaction, and so poured the water/phosphorus mix into a soup can filled with water. I thought
the soup can was clean, but later when I saw white stuff all along the edges, it looked like soup conc. Honestly!. However, the next morning, all of
the white stuff above the waterline was gone. Soup doesn't do that. I then scraped the remaining white stuff (about 90% of it was above the
water line ) into a beaker filled with water, it sank to the bottom, good. ) into a beaker filled with water, it sank to the bottom, good.
It doesn't melt at 55+deg. C, so it can't be pure phosphorus. 
When I dissasembled the apparatus, there was a white coating on most of the walls which did not react with water, and the tube inside the furnace had
some water in it from suckback. The slag was black and hard, and I'll investigate that some more tonight. There was a nice sized chunk of
phophorus (>1cc) at the spot where I had expected phosphorus to be (but could not tell w/o disassembing the apparatus), but as I was d. the a.,
that chunk disappeared.  I don't know where it went, and I wanted to
rescue and save that little guy, to keep him forever. I don't know where it went, and I wanted to
rescue and save that little guy, to keep him forever.  Now I have <1 cc of
impure white phosphorus. Hey, all I wanted it for was the challenge and thrill of making some phosphorus, and keeping it, and it looks like I've
succeeded, barely. Now I have <1 cc of
impure white phosphorus. Hey, all I wanted it for was the challenge and thrill of making some phosphorus, and keeping it, and it looks like I've
succeeded, barely. 
I want more, of course, but I think I'll take a break from that and use the same apparatus to make some sodium, working with high temps,
electricity, fire, toxic, unfamiliar, illegal, and explosive chemicals, snoopy neighbors, and firetrucks can be mentally exhausting. I feel much
safer w/ sodium. 
| Quote: | Originally posted by Marvin
Trisodium orthophosphate is not a good candidate. Sodium dihydrogenorthophosphate, or a mixture of trisodium and phosphoric acid or ammonium hydrogen
phosphates that will get you sodium dihydrogen phosphate should work much better, as the sodium metaphosphate this will form in the slow heating phase
(you need a slow heating phase to dehydrate and avoid phosphine formation) reduces much more easily.
---I tried Na3PO4 to eliminate all hydrogen from the equation, to prevent formation of phosphine, but next time I'll try sodium metaphosphate.
I would not add silica to this.
---That's what BromicAcid used, and it seemed to work there. 
Phosphorous is an oxidising agent too, excess aluminium favours phosphide formation.
---I know the Al will form phosphides.  I also know that until I use powdered
Al, the Al will not be mixed very well. Would it have helped to have a stoichiometric amount of Al? Maybe. Frankly, I don't think anyone can
be sure without a lot of tests. Besides, there was only about 1.1 times as much Al as there "should" have been. I also know that until I use powdered
Al, the Al will not be mixed very well. Would it have helped to have a stoichiometric amount of Al? Maybe. Frankly, I don't think anyone can
be sure without a lot of tests. Besides, there was only about 1.1 times as much Al as there "should" have been.
The majority of the flammable gas should be CO.
--- From the reaction chamber? I don't see how we are getting carbon to appear from sodium phosphate, silica, and aluminum. From the carbon in
the iron container???
I'm not convinced wood will get the same sorts of temperatures as charcoal.
--- Charcoal is made from wood. Charcoal is cleaner, but it's also not free. I doubt it's appreciably hotter.
I might be inclined to try a reduction with carbon using a charcoal or coke run.
---Maybe I'll try that next.
Aluminium wire cant be cost effective.
---Hmm, my total cost for reactants was $0.40. (for SiO2) I have several pounds of pure Al in the form of thick wires, picked up for free from a
construction site. Since I only needed about 12 g IIRC, this seemed fine to me.
I also think you need to scale up. 50 grams total reactants is nothing, this isnt a reaction you can do well small scale.
---If you remember what happened when I tried the sodium hydroxide/Mg thermite, you can understand why I tried this on a small scale!
Edit, something else occured to me. What stops the aluminium reducing the silica instead?
---I thought of this too. I have no answer.
[Edited on 27-8-2004 by Marvin] |
Edit- the color of the water was light yellow/orange, which I also assumed was from iron corrosion. And the reason I heated the water to boiling, it
was mostly an accident. When I added another load of wood, it didn't fit all the way down the furnace, so the lid didn't fit on tightly,
causing flames to shoot a foot long out all sides, this tends to boil water. Also, I wanted to be sure that the water was above 44 deg. C, so I
heated it a little. It heated more quickly than was anticipated.
[Edited on 28-8-2004 by Cyrus]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
There is one important matter that has been overlooked on this thread. P exists as two different allotropes: white P, which is very poisonous and
destructive to the bones (it caused "phossy jaw" in workers in old-time match factories which used the stuff, arsenic having a similar
effect), being still used in some liquid vermin poisons; and red phosphorus, which is much safer to handle and which is used in organic and inorganic
syntheses including of methamphetamine from ephedrine. However, both are slowly inflammable (hence "phosphorescent" in air, and have to be kept under water or other non-oxidizing liquid. in air, and have to be kept under water or other non-oxidizing liquid.
Which of these does your reduction processes produce?
John W.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
Phosphorus preparation will always yield white phosphorus. This is because all forms of phosphorus convert to white when heated sufficiently.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Cyrus,
"It doesn't melt at 55+deg. C, so it can't be pure phosphorus"
We dont care about the melting point, the only thing we're interested in is how well does it glow 
One matter I'm not clear on, the phosphate you used, was it trisodium orthophosphate as I assumed, or trisodium polyphosphate?
"I would not add silica to this.
---That's what BromicAcid used, and it seemed to work there"
The point of silica is to liberate the phosphate and leave a silicate. Otherwise in a carbon reduction all you can get is the phosphide (prioduction
of carbide is not going to happen). You shouldnt need this with aluminium (particually with a metaphsphate, but it should leave an aluminate), nor is
it useful in low temperature reductions.
"The majority of the flammable gas should be CO.
--- From the reaction chamber? I don't see how we are getting carbon to appear from sodium phosphate, silica, and aluminum. From the carbon in
the iron container??? "
Thats because I temperarily mislaid my brain. You are right, no carbon, but then there shouldnt be any hydrogen either to make phosphine. Did you
dehydrate the phosphate first?
"--- Charcoal is made from wood. Charcoal is cleaner, but it's also not free. I doubt it's appreciably hotter. "
Charcoal is a much hotter fuel than wood. I *think* this is because overall with wood you are producing a lot more gas (mainly water vapour) so you
are heating a larger volume of gas. This would be akin to oxygen producing a much hotter temperature than air, the nitrogen doesnt interfere, but you
have more gas to heat up with the same energy.
"---If you remember what happened when I tried the sodium hydroxide/Mg thermite, you can understand why I tried this on a small scale! "
Point taken, now I'm being unsafe. Carbon runs are almost certainly poor at that level and I'm stuck in that mindset.
JohnWW,
Phosphorous exists in a lot more than 2 forms. White, Red, Violet, Black ... I think someone said there were about 7 known forms at least. vulture
is quite correct, the vapour is in the form of P2 molecules, it will always condense to white initially under normal conditions, high pressure and
long cooking usually with a catalyst is used to make the other forms.
Red phosphorous does not glow and is virtually unreactive to air. It is not kept under water.
The term phosphorescent in english includes phosphorous, but in chemistry the more accurate term would be chemiluminescent. Phosphorescence is used
soley to describe emission of light between states of different spin multiplicities, ie it glows after you remove the source of the energy.
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
| Quote: | | You shouldnt need this with aluminium (particually with a metaphsphate, but it should leave an aluminate), nor is it useful in low temperature
reductions. |
I used SiO2 with my procedure because the references for producing phosphorus using aluminothermic reduction of phosphates stated that without silica
a large portion of the phosphorus becomes irrecoverable due to conversion to aluminum phosphide, the SiO2 reduces this and can improve yields from
about 20% to begin with to about 75%. I belive it also helps to decrease the violence of this reaction, (NaPO3)6 powdered reacting with aluminum
powder without SiO2 is almost like flash in decent amounts rendering almost impractically fast.
| Quote: | | Thats because I temperarily mislaid my brain. You are right, no carbon, but then there shouldnt be any hydrogen either to make phosphine. Did you
dehydrate the phosphate first? |
I actually think my water for phosphine formation came from the sand that I used. SiO2 does absorb water, does it have to be specially prepared to
absorb water or does it just collect on its own?
[Edited on 8/29/2004 by BromicAcid]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
I stand corrected on the addition of silica. I'm still confused as to the deeper reason though.
I understood it only absorbed water on the surface normally so fumed silica might take up a reasonable amount but sand shouldnt. Something like
silica gel is a hydrated sillic acid.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Something like silica gel is a hydrated sillic acid.
|
Silica gel is made from H4SiO4 in solution, but this acid is unstable and polymerizes to SiO2. Yes, SiO2 is actually a polymer. So it seems likely
that the clusters on the outside still have hydroxy groups attached. The smaller the gel the more hydroxylgroups you will have.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
I think the water came, as BromicAcid suggested, from any water absorbed by the very fine SiO2 or from any residual water in the sodium phosphate I
used.
The sodium phosphate was made from NH4H2PO4 and NaOH, so I think that the sodium phosphate was Na3PO4.
Edit- about charcoal vs plain wood, When I added the fresh, slightly damp, cold wood, within 0.5 to 1 min. the part of the apparatus within the
furnace would be glowing reddish orange, but when the wood had turned to charcoal, the apparatus cooled down, iirc, to a very dull red glow. 
Heat, fires, summer, hot and thick clothes, all outside. Maybe I should go for ultra LOW temps instead. 
I'll probably decide to do that about, oh, January. 
[Edited on 31-8-2004 by Cyrus]
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Tried phosphorus today using bone ash (Calcium Phosphate). I ran three attempts, a stoichiometric mix of SiO2, Al, and Ca3(PO4)2 this attempt showed
no results with my heating method, a MAPP gas torch, all reactants were very fine powders, the SiO2 was fumed silica, aluminum was 300 mesh, and the
calcium sulfate was a very fine powder. The second attempt was half way between the reduction with SiO2 and the reduction with just Al, this also was
uninitiateable with a MAPP gas torch. Finally I ran the reaction using entirely Al as the reluctant. This formed a blob at the bottom that was at
least partially composed of the phosphide as evidenced by the phosphine smell, nothing came over in the retort though.
Also I ran the reaction using sodium hexametaphosphate, silicon dioxide, and aluminum powder. All my previous attempts with (NaPO3)6 used regular
play sand as I was unable to obtain the fine SiO2 that I currently have. Therefor this one was different. It formed a solid cake at the bottom,
similar to the calcium reduction with straight aluminum. This cake was full of bubbly holes and when broken apart flared up in spots from phosphorus.
But nothing came over in the retort. I believe on my previous attempts that the SiO2 in its corse form helped to aerate the mixture and allow it to
react more thoroughly without forming that cake.
Regardless, going to try again next week possibly. I need to get a picture up of my setup still, it is pretty neat looking.
Edit: My retort
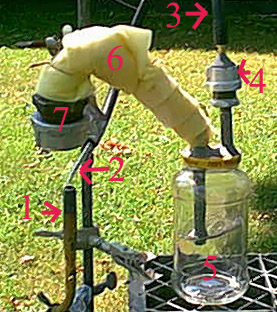
1) That is one of those hand held extension burners for soldering and such, it is hooked to MAPP gas and is held with one of those grippy claw things
that go on the ring stand. 2)That is a glass pipette that is attached to 3) A thick vacuum hose running to 4) A ball check valve to prevent suckback
of both water and air into the system. This provides the burning method of the exit gasses as the pipette runs into the flame, or at least close by
it, when it ran right into the flame the predictable result happened, the tip melted and blocked the exit. 5) A spaghetti sauce jar with two holes
put into the lid. These holes are the exit and entry points and are sealed with an epoxy around each pipe. The entry pipe pictured on the left has a
piece of screen surrounding it to prevent massive bubbles and prevent corky phosphorus. This jar is filled with water. 6) The body of the retort,
covered in fiberglass insulation to prevent phosphorus from solidifying on the inside. It is made from 1/4 inch piping except right at the reaction
vessel 7) Which is made from a 1 1/2 inch endcap connected to a 1 1/2 - 1/2 inch brushing, the brushing runs to a 1/2 inch 90 deg elbow to a short 1/2
inch pipe to adapt it to a 45 deg 1/2 - 1/4 adapter.
Simple with only a few joints and prevents suck back and exit gasses are held in check. I like it

[Edited on 9/1/2004 by BromicAcid]
|
|
|
Cyrus
Hazard to Others
  
Posts: 397
Registered: 24-4-2004
Location: Ancient Persia
Member Is Offline
Mood: No Mood
|
|
Are you sure that the "jam jar" will not implode under vacuum? That would be unfortunate.  Might a metal container be better? Might a metal container be better?
In order to prevent suckback, how about this?
Well the picture isn't working, my idea was to have a tee connection in between the heated part and the water with a ball valve connected to
that, on the other side of the ball valve, a balloon filled with an inert gas. When heating is done, slowly open the ball valve.
[Edited on 2-9-2004 by Cyrus]
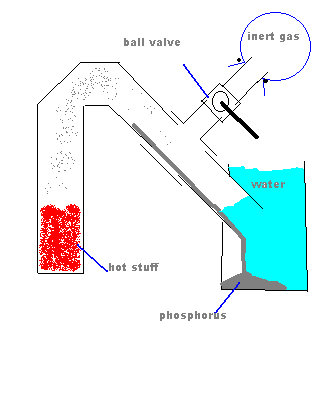
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Your design looks nice but with the scale of my design (my reaction vessel is only 36 cm 3) There really isn't enough area to generate a
significant vacuum. Besides, my ball valve check is not totally air tight, it is home-aid, and the spaghetti jar is probably not air tight either, I
am not afraid of it imploding, although worse things have happened 
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Alternative phosphorus production using an interesting mechanism I saw the other day.
Zn3P2(s) + HCl(aq) ---> ZnCl2(aq) + PH3(g)
PH3(g) + Ni(CH3COO)2(aq) ---> P(x)Ni(x)(s) + xCH3COOH(aq)
Commercially available zinc phosphide in the form of mole killing pellets is reacted in an airtight vessel with concentrated hydrochloric acid at a
controlled rate. The exit gasses consisting mostly of phosphine are lead though a concentrated solution of a soluble nickel salt. Phosphine reduces
the nickel forming nickel-phosphorus 'alloys' of variable composition. These alloys are filtered from the solution and subject to treatment
with dillute potassium dichromate/sulfuric acid solutions to yeild free phosphorus which is melted under water to consolidate it into one blob.
I was thinking of this due to a passage that I read the other day that bubbling phosphine though soluble nickel salts results in the formation of
insoluble nickel alloys of high phosphorus content, under suitable conditions true alloys can be formed. The dichromate sulfuric acid is the normal
procedure to wash phosphorus and hopefully it would dissolve out the nickel given time. According to my calculations one container of mole pellets
(250 g) should yield 1 g of phosphorus, but it's an alternative method. However ... the extreme toxicity of phosphine (and its long term
effects) make this method an adventure that I would not be willing to take.

|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Actually that is a method I'd happy to undertake as long as it's done outside, and upwind...
Sounds like an easy method otherwise!
Apart from this pellet - are there any other sources, or ways, to get Zn3P3, or other alternativve phosphides?
I mean, think about it, do you think sublimating phosphorous is much more pleasant, particulalry at the temperatures normally required?
Also - what is the mechanism of it? i.e. the reduction of PH3 to P, forming this 'alloy'?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Unless you use a gas mask, or better still breathing apparatus like what firefighters use. The toxic mechanism of PH3 is similar to that of CO, H2S,
HCN, (CN)2, azides, etc, due to its strongly complexing Fe in hemoglobin. In fact, it may be more toxic than even H2S.
I think Zn3P2 is also used for killing rats and especially rabbits, by putting the pellets into their burrows.
John W.
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
I chose Zn3P2 because it is the most widely avalible, as John WW said it is for killing rabits and other wildlife, moles like I said. Around here it
comes in 250 g containers and is between 2 and 4% Zn3P2.
The mechanism is just phosphine reducing the nickel and in the process being oxidized to elemental phosphorus.
2PH3(g) + 3Ni2+(aq) ----> Ni3P2(s) + 6H+(aq)
That's just an approximate equation, I will re-look up the reference tommorow since there is intrest but it's not a phosphide that is
formed, it is a mixture, so that formula would not be accurate. The book stated that the mixture formed is of a highly variable composition depending
on condition. Will post more tommorow.
|
|
|
BromicAcid
International Hazard
    
Posts: 3266
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Taken form "Chemical Elements and their Compounds" Vol I 1962 Sidgwick pg 730
| Quote: | | Phosphine will reduce nickel salts in aqueous solution, forming alloy-like P-Ni compounds with 0.4 - 0.1 P to 1 Ni : under special conditions,
definite phosphides Ni3P, Ni2P, and NiP are obtained. 457 |
457 R. Scholder, A. Apel, and H.L. Haken, Z. anorg. Chem. 1937, 232, 1.
|
|
|
Theoretic
National Hazard
   
Posts: 776
Registered: 17-6-2003
Location: London, the Land of Sun, Summer and Snow
Member Is Offline
Mood: eating the souls of dust mites
|
|
Zinc shall save you
I've had an idea. Zinc could be the perfect reducing agent. Look what happens if you have molten Na3PO4 and you throw in a gob of zinc (in
stoichiometric proportion, of course).
First zinc reduces PO4--- to P---:
PO4--- + 4Zn => P--- + 4ZnO.
The thus formed oxide film on zinc is dissolved away by the phosphate ions:
ZnO + 2PO4--- => ZnO2-- + P2O7----,
then when ortophosphate ions run out its:
ZnO + P2O7---- => ZnO2-- + 2PO3-.
Of course all of the PO4--- turns into PO3- and P--- before the zinc runs out, then phosphide and metaphosphate start conproportionating:
5P--- + 12PO3- => 8P + 9PO4---,
PO4--- reacts with ZnO as was shown above, the exposed zinc surface reduces some more PO4---, and so you again have phosphide and ortophosphate, they
react and the cycle repeats untill zinc runs out and you have a solution of ZnO in molten Na2ZnO2 in your vessel, and dough* (sorry, white phosphorus)
in the condenser. The advantages are that the zinc can be a gob instead of a powder, it's a much less vigorous reducer than Al and so the
reaction can't get out of hand, there are two components instead of three (phosphate and zinc as opposed to phosphate, Al and SiO2), the reaction
isn't stopped by a tough oxide layer, is faster and goes much nearer to completion. The disadvantage is that aluminium is very easy, getting
sizeable amounts of zinc is harder.
*dough - taken from Stupid White Men by Michael Moore, where he says: More dough for YOU! meaning money, I meant phosphorus  . .
[Edited on 18-9-2004 by Theoretic]
|
|
|
Al Koholic
Hazard to Self
 
Posts: 98
Registered: 2-12-2002
Member Is Offline
Mood: Seeking ligand
|
|
To boot
Acquiring Zn has been discussed in another thread but I'd just like to point out that by melting a few bucks worth of US pennies one can obtain
quite a bit of Zn. Just scrape off the slag formed after the melt to get reasonably pure material. I also doubt impurities will really matter.
|
|
|
| Pages:
1
2
3
4
5
6
..
60 |
|