| Pages:
1
2
3
4
5
6
..
20 |
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
| Quote: | Originally posted by Kalle anka
Man! You guys must have written essentially all methods possible to make aliphatic aldehydes. But what comes to novel reactions here is something that
may be of some interest for you crazy people out there!
Now talking about acetaldehyde i want to tell you about an experiment of mine. One night i dreamt about several things but i woke up when it crossed
my mind. I wanted to make copper salts from metallic copper but as you guys know its reduction potential is higher than hydrogen, ie. acids wont bite
on it. A classical example in vitually all inorganic chems books i've seen is the demonstration of HNO3 as an oxidising acid. I have no access to
HNO3 nor H2SO4, so the reason i woke up suddenly was that i realised (omg im a n00b) that the NO3 ion is reduced.
My point is that i oxidised metallic copper which i took from some net cords and other cables with NaNO3 and HW grade 30% HCl. It needed some external
heating to start but then proceeded with a violent continuous exhaust of NO2 which settled as a reddish-brown toxic fog on the floor  Later i dreamed i wanted to see if i could do something else so i just grabbed what
we here in Sweden call "spolarvätska", dont have the english word for it but it is a solution of ethyl alcohol in water which is used in
the cars to rinse the main window. You could use any other available ethyl alcohol for this purpose. Later i dreamed i wanted to see if i could do something else so i just grabbed what
we here in Sweden call "spolarvätska", dont have the english word for it but it is a solution of ethyl alcohol in water which is used in
the cars to rinse the main window. You could use any other available ethyl alcohol for this purpose.
Anyway, as the relatively bearable chlorine like smell of NO2 could not have been enough, this shit made an awful pungent strong f*cking apple odour
even in my dream, which took a hell of a long time to exit. No appetite for apples 
As i made this in a ordinary 250ml rbf, all shit boiled off from the warm (~80*C) NaNO3+HCl solution and really just gave me a bad day. If i really
wanted to look at this possibility to make acetaldehyde, i would have set up a distillation apparatus and added 96% ethyl alcohol through a claisen
dropwise to the warm solution of HCl and NaNO3. I would also have liked to keep water through condensor cold and possibly placed the reciever in an
ice bath. But until then i think i just wake up and do something constructive.
Take care and have fun  |
I don't get it, what did you make?
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
He dropped copper oxide into denat. alc. and got some apple like smell.
chemoleo wrote: | Quote: | the smell was definitley there, but it's hard to separate the aldehyde from the rest.
|
And thats exactly the point. We have a trillion easy methods to produce an apple smell from alcohol - actually about anything what hase some oxidising
properties does this. But one gets no acetaldehyde as the the smell seems to be present with minute amounts of aldehyde already in special when this
is in solution.
Woth to mention might be that the cinnamaldehyde decomposition with bases produces equimolar amounts of benzaldehyde and acetaldehyde.
|
|
|
Kalle anka
Harmless

Posts: 15
Registered: 10-6-2005
Location: Scandinavia
Member Is Offline
Mood: decomposing
|
|
damn it.. i saw that the Hcl + NaNO3 mixture was a good oxidiser mixture. when NO3 oxidzes something its reduction potential is 0.96V, lower than
Cr2O7, ie. its a weaker/milder oxidiser than dichromate.
I added ethanol to this mixture and got the damn acetaldehyde that is the topic of this thread geezers! What else with an apple like odour could
i've made that would fit under this topic?
|
|
|
Organikum
resurrected
    
Posts: 2342
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
As told before: Some acetaldehyde was produced - this is easy. But I doubt that it was produced in reasonable amounts.
Separate - measure.
Apple smell is already present with traces of acetaldehyde and it is no prove at all.
By now you have not made acetaldehyde but a mixture with an apple like smell.
/ORG
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
The main problem with the production of acetaldehyde from ethanol is that when you use water as a solvent, you're always wasting aldehyde by
conversion to acetic acid.
The easiest solution to prevent this is using aprotic polair solvents, but they are usually expensive, hard to get and/or toxic/nasty.
Now, a suspension of CrO3 in aceton is being used in the Jones-oxidation, this might be interesting. Solid CrO3 embedded in graphite can also be used
to specifically convert alcohols to aldehydes (Lalancette reagent).
Maybe CrO5 in ether solution is also an option, but this would require caution, as it is a very powerful oxidizing agent.
Separation of ether/aldehyde would also cause problems.
[Edited on 15-6-2005 by vulture]
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
| Quote: | Originally posted by vulture
Maybe CrO5 in ether solution |
Decavalent? 
Hm, not really sure what else you meant... CrO3 is the highest valence anhydride (or rather Cr2O6) I'm aware of.
Edit: I'll be darned, you can isolate perchromate?
Tim
[Edited on 6-16-2005 by 12AX7]
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
No, hexavalent. It contains two peroxogroups.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
| Quote: |
I added ethanol to this mixture and got the damn acetaldehyde that is the topic of this thread geezers! What else with an apple like odour could
i've made that would fit under this topic? |
I don't know, I never smelled acetaldehyde. Btw, did you purify/isolate it, what was the yield? Unless these detais are known your post is
meaningless.
[Edited on 16-6-2005 by Sandmeyer]
|
|
|
chloric1
International Hazard
    
Posts: 1159
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
| Quote: | .
I am not sure about this being a good route. I remember doing oxidation experiments with H2O2, and the smell was definitley there, but it's hard
to separate the aldehyde from the rest.
|
I hear ya but what about separation with concentrated bisulfite solution? This should isolate most small molecule carbonyls from alcohols and the
like. THen you only need to add some dilute sodium bicarbonate or HCL to break down the bisulfite.
Fellow molecular manipulator
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Anyone having vanadium pentoxide on hand might try adding hydrogen peroxide
to a stirred suspension of the vanadium pentoxide in warm ethanol . I'm thinking
pervanadic acid might form in sufficient amount to oxidize the ethanol to acetaldehyde which should volatalize as it is formed . The vapor could be
condensed
and the acetaldehyde isolated as a liquid , or the vapors could be conducted directly to whatever subsequent reaction where the acetaldehyde is needed
and introduced through a bubbler or dispersion tube .
Here's a link for a similar reaction .
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&a...
I found a citation for a journal reference regarding the production of acetaldehyde from ethanol by vanadium peracid . This journal article should be
given a look , perhaps added to the literature request list .
Conte, V.; Di Furia, F.; Modena, G. J. Org. Chem. 1988, 53, 1665
EDIT : It appears that the citation was in error and the more needed reference is an earlier article by the same researchers found here :
Bortolini, O.; Conte, V.; Di Furia, F.; Modena, G. Nouv. J. Chim. 1985, 9, 147-150
The JOC article is only a followup relating to the production of acetone from isopropanol . But the original article describes the details for the
reaction conditions , and the production of acetaldehyde from ethanol . The pertinent
reference is likely in Italian , so translation
will be needed if an English version is not
already a parallel publication .
Evidently there is an English version of the journal , published as
" New Journal of Chemistry "
[Edited on 17-6-2005 by Rosco Bodine]
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
After reading about oxidation of alcohol with H2O2 using V and Mo as catalysts, I decided to give it a try.
First I did a few small experiments in test tubes to see what effect different catalysts had on peroxides ability to oxidize ethanol to acetaldehyde.
It was suggested that simply adding a catalyst to the mix such as KmNO4 forming O2 and possibly nasent O might do the trick.
I made a simple mix of 50ml of 95% ethanol and 50ml of H2O2 50%. I split this equally into 4 test tubes.
To the first I added ~.01gm V2O5 - there was a slight reaction that subsided in a few seconds to a stable solution.
To the second, four 1mm platinum catalyst beads. - there was immediate evolution of O2 that continued is a stable manner.
The third ~.005gm KMnO4 - a fairly rapid evolution of O2 began and at one point rose up in the tube but then settled down to a constant fizzing.
the fourth ~.008gm MnO2 - same as #1
These were loosely capped and left to sit for 2 hours. After uncapping there was only the scent of ethanol in #2 and #3. In #1 and #4 there was a
strong smell of CH3CHO. I seriously doubt the simple mixing of H2O2 and CH3CH2OH. Even with the addition of something like KMnO4 or MnO2 to cause the
H2O2 to decompose probably would not form any appreciable quantities of aldehyde.
Experiments with V2O5:
96ml C2H5OH and .1 gm V2O5 and a few teflon boiling stones were placed in a 500ml 3 neck flask equipped with an addition funnel, thermometer and a
jacketed condenser set for reflux then connected to a Graham condenser being cooled with 5º circulated water leading into a receiver cooled in an ice
bath.
http://img80.imageshack.us/img80/650/setup1by.jpg
100 ml of H2O2 50% was placed in the addition funnel. 25 ml of peroxide was slowly added to the alcohol V2O5 soln dropwise as the flask was being
warmed. At about 40~ the liquid began a slight boil.
http://img80.imageshack.us/img80/2905/startofrxn2tx.jpg
After temperature reached 50º the mantle was shut off. The temperature continued to rise and the liquid began a medium boil at about 60º. At this
point a distillate started coming over slowly and the top of column was reading ~25º. H2O2 was again added in about the same rate as the distillate
was coming off. I kept cold tap water flowing through the reflux in an attempt to keep the reaction under control. When the temperature went over
80º, I pulled out the mantle and set the flask on rings and began to flush the outside with tap water to throttle the thing back.
http://img80.imageshack.us/img80/2406/runaway5vi.jpg
This reaction continued for about another 1/2 hour with continuos cooling both in the column and on the outside of the flask. All the peroxide was in
by now. Meanwhile the temperature at the head of the column was held below 40º as best as possible. The vigorous action began to slow. At this point
the mantle was put back in the game to keep the reflux going as I thought there might still be some unreacted ethanol. Even with additional heating
the thing was still slowing down and some interesting color changes happened.
http://img80.imageshack.us/img80/787/yeltored3eo.jpg
Then back to yellow with a tinge of green.
http://img80.imageshack.us/img80/5606/yelgr21yy.jpg
And finally to green after she was tired out.
http://img80.imageshack.us/img80/8018/green9kj.jpg
After distilling the product once more, coming over at 21º to 23, final total was 18 grams of acetaldehyde as confirmed by FTIR. Also something I
didn't expect, in the higher boiling fraction of the original distillate was found ethyl formate.
http://img80.imageshack.us/img80/66/ethform7zu.jpg
I also ran the green leftovers and found more ethyl formate as well as ethyl acetate. Which makes sense as it smelled strongly of acetic acid.
I have tried this reaction now 3 times.
The first as above by adding the H2O2 slowly to the mix of C2H5OH and V2O5.
The second, I tried adding the EtOhV2O5 to the warmed H2O2 in the flask. And third carefully mixing the V2O5 with the H2O2 before and then adding to
the flask,
http://img80.imageshack.us/img80/2408/mix2zd.jpg
warming then slowly adding the EtOh. All in all it was about a draw, with the best yield in the 3rd setup of a whopping 28.02gm of product. I think I
wouldn't do this method again.
Final note:
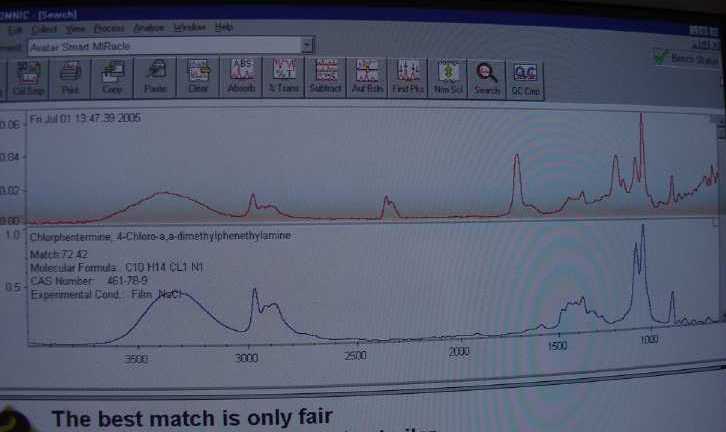
I did do this reaction a fourth time, similarly to the third run above but in the H2O2/V2O5 mix I added about 3ml of Hcl in an attempt to lower the ph
and improve the yield. What I got was a yield of possibly something else…The FTIR said it could be "methyl vinyl ether -alt- maleic acid mono
ethyl ester" Or the pic below… it says only a fair match but to be sure that sample was destroyed immediately!!!
[Edited on 2-7-2005 by ordenblitz]
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
FYI, you can take screenshots by pressing the PRINTSCREEN button then pasting in your favorite image editor. 
Tim
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Interesting experiment !
The yellow to red color is pervanadic acid ,
or perhaps an unstable ethanol pervanadic acid complex .
That colored intermediate is consumed in the oxidation of the alcohol and then regenerated by the reaction with the incoming hydrogen peroxide . It
is possible that if additional ethanol and hydrogen peroxide were simultaneously added in correct proportions to the warm red mixture at the correct
temperature, the reaction may possibly continue steadily at a rate regulated by the speed of the addition , until a point of dilution by accumulating
water from the H2O2 quenches the reaction . How far it might continue would depend on the concentration of H2O2 being used .
The ratio of acetaldehyde to higher oxidation products likely decreases with
temperature , so the yield may increase
running the reaction at the lowest acceptable rate where the reaction still proceeds . The amount of catalyst in
the mixture may also have some bearing .
There is likely an optimum concentration for a given temperature and reaction rate . Buffering the reaction mixture
is another possibility if a particular pH favors the oxidation for acetaldehyde .
Anyway , the use of pervanadic acid looks like a valid method for acetaldhyde , for those not inclined to go the route using tube furnaces .
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Your 95% etOH being denatured with methanol would explain the ethyl formate. Just having the methanol being oxidized to formic acid then undergoing
an esterification reaction with the ethanol.
Interesting experiment.
[Edited on 2-7-2005 by rogue chemist]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
28 grams of acetaldehyde from 96 ml ethanol is a 39% of theoretical yield .
Definitely on the right track and yield could probably be increased considerably with some variations on the temperatures and probably the pH .
Perchromic acid should behave similarly ,
and may be a bit less active so it may be worth trying if overoxidation is in part responsible for the low yield via pervanadic acid oxidation .
That New Journal of Chemistry article may shed some light on the reaction conditions which are most favorable .
I know that sodium dichromate solution acidified by gradual addition of dilute sulfuric acid can also be used for the oxidation of different materials
to aldehydes . I wonder if such a chromic acid solution could not be enhanced to function as a perchromic acid regenerating
reagent simply by gradually adding hydrogen peroxide to the reaction mixture . A much smaller amount of dichromate and sulfuric acid could be used
simply to start the reaction , since the peracid regenerates .
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
12AX7,
Thanks for that suggestion. It will save me time screwing around with the camera. I am a Mac guy and don’t know all the cleaver pc stuff.
Roscoe,
You are right on target about the color changing with the amount of H2O2 present. I tested this with some spent remains and was able to easily change
the dark green color back to dark red then back to reddish yellow again. One would need only to reduce the water and begin again. I did some small
scale tests again today, adding drop wise, H2O2/ethanol mix to V2O5 in a slight amount of etoh. The reaction is more controllable. One needs only to
be able to cool the reaction to hold about 40deg., if it goes much higher and you can really detect acetates being formed. The reaction is fairly slow
at 40 though and it might take multiple hours to complete. I would like to explore your perchromic idea further; I think it’s a good one.
Rogue chemist,
I used grain alcohol so there should have been no methanol present. I was puzzled by the formate as well.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Probably to get the best yield of acetaldehyde may require operating the reaction just warm enough for the catalyst to stay in active regeneration ,
and also boil off the acetaldehyde . The temperature for regeneration activity is probably going to be the limiting factor ,
and that will be the parameter where the concentration of the catalyst will be a real factor in optimizing the conditions for acetaldehyde . I think
that once the right conditions are found , the reaction could be set to run at a steady but slow rate ,
moderated by a shallow depth of flowing tap water , which is a fairly constant temperature , and then control the reaction at some drops per minute
count found to keep things " in the groove " so the reaction could run unattended and
deliver the acetaldehyde to a receiver bottle in a styrofoam ice bucket . An old IV pump would really be ideal for this reaction , as you could run
separate metered feeds of alcohol and H2O2 .
With a digital temp sensor and a parallel port on the IV pump , you could write a little control program and have the PC babysit the reaction as
process controller ,
rate regulator type of setup . Put the entire apparatus in a footlocker with wheels and you have a portable acetaldehyde factory  Add a few extra reaction lines and another reaction chamber with some formaldehyde
and lime , and the box will be shitting pure pentaerythritol everywhere it goes Add a few extra reaction lines and another reaction chamber with some formaldehyde
and lime , and the box will be shitting pure pentaerythritol everywhere it goes     You'll have to keep behind it with a shovel to keep it from getting too deep You'll have to keep behind it with a shovel to keep it from getting too deep

|
|
|
mantis
Harmless

Posts: 38
Registered: 17-7-2005
Member Is Offline
Mood: No Mood
|
|
A good and clean way to produce acetaldehyd is the oxidation of lactic acid by H2O2:
CH3-CH(OH)-COOH+H2O2 ---> CH3-CHO+CO2+2H2O
To have a faster reaction you can heat it up a bit.
When you boil lactic acit you get a mix of acetaldehyd and formic acid. As cat. you can use sulphuric acid.
CH3-CH(OH)-COOH cat. or heat--->CH3-CHO+HCOOH
[Edited on 20-7-2005 by mantis]
|
|
|
ordenblitz
Hazard to Others
  
Posts: 259
Registered: 18-7-2004
Location: Northwest
Member Is Offline
Mood: Bohr'd
|
|
HCCH + H2O to CH3CHO
I have been wanting to get the time to try production of acetaldehyde by passing C2H2 through a dilute solution of H2SO4 containing a small amount of
HgSO4. Today I took the time.
105 ml. H2O
15 ml. H2SO4 96%
0.5 gm. HgSO4
Were mixed together and placed in a 250 ml. kjeldahl flask and warmed in a water bath to 60 deg C
In the flask was placed a fine glass airstone bubbler fitting with exit jacket. I connected the outlet via hose to a cooled graham condenser that lead
into a collection flask. A commercial welding acetylene tank was connected through the regulator to the airstone inlet.
After opening the tank and starting the gas flow slowly, very soon a strong acetaldehyde smell could be detected coming from the collection flask. It
was very strong and somewhat dissimilar to the smell I am used to from the CH3CHO made from ethanol. Usually you end up with a mix and get the smell
of both.
If I ran the gas in strongly, I could also smell the C2H2 but when running it slowly all I could smell was the sweet strong stink.
Ok I thought, we are off to a good start. So I turned the chiller flowing through the condenser down to 5C and waited.. and waited.. and waited! Come
on dammit, could I just get one stinking drop? Nope!
I also discovered that this reaction is exothermic. You only have to get the temp up to 60 before starting then you need to apply cooling. I let the
temperature rise to 80 in the beginning and the production of acetaldehyde ceased. I wasn’t aware that I would need cooling.
Ok so this method definitely works but…. You need a big reaction chamber, at least a very long column and a lot of contact area. You have to be able
to move a lot of gas to make a small amount of liquid. My bad as I didn’t take the time to crunch the numbers on this before starting. To make this
workable for lab scale production I am guessing you need a column maybe 8 cm in diameter and possibly 1 to 2 meters in length.
Sorry, no pics today as I forgot to charge my cam batteries.
[Edited on 8-8-2005 by ordenblitz]
|
|
|
praseodym
Hazard to Others
  
Posts: 137
Registered: 25-7-2005
Location: Schwarzschild Radius
Member Is Offline
Mood: crazy
|
|
Acetaldehyde can also be prepared by oxidising EtOH or from acetic acid by dry distillation of calcium acetate with sodium formate, i think.
|
|
|
froot
Hazard to Others
  
Posts: 347
Registered: 23-10-2003
Location: South Africa
Member Is Offline
Mood: refluxed
|
|
I found out after many attempts that the setup for making acetaldehyde is crucial. In my case I used the ethanol method but the condensing appauratus
should be similar.
The only time when I got results was when I set up my condensor vertically as in reflux mode. I let normal cold tap water run through the jacket.
From the condensor ran a 8mm nb tube of about 2m length through a bucket of water full of ice. The receiver was also kept in ice.
I feel it is imperative that the vapours leaving the reaction are chilled properly. I used the condensor in upright position to aid chilling and
remove any water vapour before the acetaldehyde is precipitated.
I thought this might help with your acetylene method.
We salute the improvement of the human genome by honoring those who remove themselves from it.
Of necessity, this honor is generally bestowed posthumously. - www.darwinawards.com |
|
|
DeAdFX
Hazard to Others
  
Posts: 339
Registered: 1-7-2005
Location: Brothel
Member Is Offline
Mood: @%&$ing hardcore baby
|
|
| Quote: | Originally posted by Polverone
According to The Old References, pyruvic acid itself is prepared by distilling tartaric acid with KHSO4 at 200-250 C. And potassium hydrogen tartrate
is easily available (though a bit expensive) from grocery stores. Find a place that sells in bulk; I can get it for about $13/kg. If bought in a
little spice bottle it's probably more like $50/kg. Now you don't need to order from the net or expensive health food stores at all!
Of course, this depends on how pure your acid must be... some old methods leave products that are difficult to purify, or else the yields are bad.
|
Could other HSO4- salts be used such as ammonia or is potassium the only salt that will work?
Im guessing potassium tartrate and sulfuric acid would work too?
|
|
|
markgollum
Hazard to Self
 
Posts: 53
Registered: 21-2-2004
Member Is Offline
Mood: No Mood
|
|
The hydration of acetylene.
ordenblitz, the temperature that you used for the hydration of acetylene was far to hot!, yields drop to below 50% at temperatures above 50degC.
If I were going to try this route to acetaldehyde I would use the method where the catalyst is a moist paste of sodiumbisulfate and HgO (reacts to
form the sulphate).
The advantage of using this catalyst is that the acetaldehyde produced is polymerized to paraldehyde in the reaction flask and later poured off. (see
the pages I scanned)
|
|
|
markgollum
Hazard to Self
 
Posts: 53
Registered: 21-2-2004
Member Is Offline
Mood: No Mood
|
|
Sorry, I forgot the attachment.
[Edited on 6-6-2006 by markgollum]
edit (  I can't seem to add my attachment, I guess 1.55MB (6 pages) is to much?, I can't seem to add my attachment, I guess 1.55MB (6 pages) is to much?,
 . ) . )
[Edited on 6-6-2006 by markgollum]
|
|
|
Elawr
Hazard to Others
  
Posts: 174
Registered: 4-6-2006
Location: Alabama
Member Is Offline
Mood: vitriolic
|
|
Apple like smell?... reminds me of high-school chem lab when we prepared simple esters. Really intense fruity smells of various kinds that lingered
for weeks, it seemed. Could your reaction have oxidized some of your Etoh to ethanoic acid? What does ethyl acetate smell like?
|
|
|
| Pages:
1
2
3
4
5
6
..
20 |